
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
As confidentially submitted with the U.S. Securities and Exchange Commission on February 10, 2021
This draft registration statement has not been publicly filed with the U.S. Securities and Exchange
Commission and all information herein remains strictly confidential.
Registration No. 333-___________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
|
GROVE, INC. |
|
(Exact name of Registrant as specified in its charter) |
|
Nevada |
|
5900 |
|
83-3378978 |
|
(State or other jurisdiction of incorporation or organization) |
|
(Primary Standard Industrial Classification Code Number) |
|
(I.R.S. Employer Identification Number) |
1710 Whitney Mesa Drive
Henderson, NV 89014
(701) 353-5425
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Andrew J. Norstrud
Chief Financial Officer
1710 Whitney Mesa Drive
Henderson, NV 89014
(701) 353-5425
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Please send copies of all communications to:
|
Mark Lee, Esq. Greenberg Traurig, LLP 1201 K Street, Suite 110 Sacramento, CA 95814 Tel: (916) 868-0630 Fax: (916) 448-1709 |
|
Ross Carmel, Esq. Philip Magri, Esq. Carmel, Milazzo & Feil LLP 55 W 38th Street, 18th Floor New York, NY 10018 Tel: 212-658-0458 Fax: 646-838-1314 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
|
Proposed Maximum Aggregate Offering Price(1) |
|
|
Amount of Registration Fee(3) |
| ||
|
|
|
|
|
|
|
| ||
|
Common Stock, par value $0.001 per share (2)(3) |
|
$ |
|
|
|
$ |
|
|
|
Underwriters’ Warrants to purchase Common Stock (4) |
|
|
-- |
|
|
|
-- |
|
|
Shares of Common Stock, issuable upon the exercise of the Underwriters’ Warrants (5) |
|
|
|
|
|
| ||
|
Total |
|
$ |
|
% |
|
|
| |
______________
|
(1) |
Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”) |
|
(2) |
Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
|
(3) |
Includes shares which may be issued upon exercise of a 45-day option granted to the underwriters to cover over-allotments, if any |
|
(4) |
In accordance with Rule 457(g) under the Securities Act, because the shares of the Registrant’s Common Stock underlying the Underwriters’ Warrants are registered hereby, no separate registration fee is required with respect to the warrants. |
|
(5) |
Pursuant to Rule 457(g) under the Securities Act, the registration fee is determined pursuant to the price at which a share subject to the Underwriters’ Warrants may be exercised, which is equal to 110% of the public offering price per share. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this Registration Statement shall become effective on such date as the U.S. Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated ___________
PRELIMINARY PROSPECTUS

GROVE, INC.
____________ Shares of Common Stock
This is the initial public offering of our Common Stock. We currently expect the initial public offering price to be between $ and $ per share of our Common Stock.
Prior to this offering, there has been no public market for the shares of our Common Stock. We intend to apply to list the shares of our Common Stock on [________] under the symbol “[_____]”.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, and a “smaller reporting company” under applicable federal securities laws and as such are eligible for reduced public company reporting requirements. See “Prospectus Summary— Implications of Being an Emerging Growth Company and Smaller Reporting Company.”
Investing in our Common Stock involves a high degree of risk. Before buying any shares, you should carefully read the discussion of material risks of investing in our Common Stock in “Risk Factors” beginning on page 13 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share |
|
|
Total (1) |
| ||
|
Initial public offering price |
|
$ |
|
|
$ |
| ||
|
Underwriting discounts and commissions(2) |
|
$ |
|
|
$ |
| ||
|
Proceeds to us (before expenses) |
|
$ |
|
|
$ |
| ||
___________
(1) Assumes no exercise of the over-allotment option by the underwriters.
(2) We have agreed to reimburse the underwriter for certain expenses. See “Underwriting” for additional information regarding compensation payable to the underwriters.
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
This offering is being conducted on a firm commitment basis. The underwriters are obligated to take and pay for all the Common Stock offered by this prospectus if any such shares are taken.
We have granted each of the underwriters an option, exercisable for 45 days from the date of this prospectus, to purchase up to ______________ additional shares of common stock at the public offering price, less the underwriting discounts and commissions to cover over-allotments, if any. If this over-allotment option is fully exercised, the Company will receive an additional $ ______ and total proceeds to us will be $ .
The underwriters expect to deliver the shares to purchasers on or about , 2021, through the book-entry facilities of The Depository Trust Company.
Book-Running Manager
KINGSWOOD CAPITAL MARKETS
division of Benchmark Investments, Inc.
The date of this prospectus is , 2021.
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
3 |
| |
|
|
9 |
| |
|
|
11 |
| |
|
|
13 |
| |
|
|
26 |
| |
|
|
26 |
| |
|
|
27 |
| |
|
|
27 |
| |
|
|
28 |
| |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
31 |
|
|
|
43 |
| |
|
|
58 |
| |
|
|
62 |
| |
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND CORPORATE GOVERNANCE |
|
65 |
|
|
|
66 |
| |
|
|
67 |
| |
|
|
69 |
| |
|
|
71 |
| |
|
|
74 |
| |
|
|
79 |
| |
|
|
79 |
| |
|
|
79 |
| |
|
|
F-1 |
|
You should rely only on the information contained or incorporated by reference into this prospectus or any free writing prospectus prepared by us. We have not authorized any other person to provide you with information different from or in addition to that contained in this prospectus or any free writing prospectus prepared by us, and we take no responsibility for any other information that others may give you. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. You should assume that the information appearing in this prospectus and any free writing prospectus prepared by us is accurate only as of the date on its respective cover, regardless of the time of delivery of this prospectus or any free writing prospectus or the time of any sale of shares of our Common Stock. Our business, financial condition, results of operations and prospects may have changed since those dates.
We have proprietary rights to trademarks, trade names and service marks appearing in this prospectus that are important to our business. Solely for convenience, the trademarks, trade names and service marks may appear in this prospectus without the ® and ™ symbols, but any such references are not intended to indicate, in any way, that we forgo or will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, trade names and service marks. All trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
| 1 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
STATEMENT REGARDING INDUSTRY AND MARKET DATA
The industry and market data in this prospectus are based on the good faith estimates of management, which estimates are based upon our review of internal estimates, research studies and surveys, independent industry publications, and other publicly available information. Industry publications and research studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. These data involve a number of assumptions and limitations, and investors are cautioned not to give undue weight to such estimates. Although neither we nor the underwriters have independently verified the accuracy or completeness of any third-party information, we believe that the information from these publications and studies included in this prospectus is generally reliable, and the conclusions contained in the third-party information are reasonable.
Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and additional uncertainties regarding the other forward-looking statements in this prospectus. In addition, projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors” and elsewhere in this prospectus. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
| 2 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
PROSPECTUS SUMMARY
This summary highlights information described more fully elsewhere in this prospectus. Because it is a summary, it may not contain all of the information that is important to you. Therefore, you should read this entire prospectus carefully, including, in particular, the sections entitled “Risk Factors” beginning on page 13 of this prospectus and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 33 of this prospectus, and our consolidated financial statements and related notes, before making an investment decision. Some of the statements in this summary and elsewhere in this prospectus constitute forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.” In this prospectus, unless the context otherwise requires, references to “we,” “us,” “our,” the “Company,” or “Grove” refer to Grove, Inc. and its consolidated subsidiaries.
Our Company
We are in the business of developing, producing, marketing and selling raw materials, white label products and end consumer products containing the industrial hemp plant extract, Cannabidiol (“CBD”). We sell to numerous consumer markets including the nutraceutical, beauty care, pet care and functional food sectors. We seek to take advantage of an emerging worldwide trend to re-energize the production of industrial hemp and to foster its many uses for consumers. The development of products in this highly regulated industry carries significant risks and uncertainties that are beyond our control. As a result, we cannot assure that we will successfully market and sell our planned products or, if we are able to do so, that we can achieve sales volume levels that will allow us to cover our fixed costs.
The Company primarily conducts its business operations through its wholly-owned subsidiaries: Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company; One Hit Wonder Holdings, LLC, a California limited liability company; SWCH LLC a Delaware limited liability company; Trunano Labs, Inc., a Nevada corporation; Infusionz, LLC a Colorado limited liability company; and Cresco Management, LLC, a California limited liability company.
Historically cultivated for industrial and practical purposes, hemp is used today for textiles, paper, auto parts, biofuel, cosmetics, animal feed, supplements and much more — an impressive scope for such a historically misunderstood and restricted commodity. The market for hemp-derived products is expected to increase exponentially over the next five years, and we believe Grove is well positioned to be a dominant player in the hemp industry.
In the U.S., hemp products are generally regulated by the Agriculture Improvement Act of 2018 (United States) (the “2018 Farm Bill”). Consequently, the Company processes, develops, manufactures, and sells its products pursuant to the 2018 Farm Bill. CBD products produced and sold by Grove constitute hemp under the 2018 Farm Bill.
In addition, through one of our wholly owned subsidiaries, we produce primarily business-to-business CBD related trade shows in the United States and were looking to expand prior to the COVID-19 pandemic. The trade shows have been profitable and allow Grove to market its own CBD products and services while also increasing the awareness of the expanding CBD market to the public.
Market Opportunity
The industrial hemp market is projected to grow at a CAGR of 34% from USD 4.6 billion in 2019 to USD 26.6 billion by 2025. The growth of this market is attributed to the increased consumption of hemp-based products due to their various health benefits and increased incidences of diseases such as epilepsy and other sleep disorders. However, the complex regulatory structure for the usage of industrial hemp in different countries is expected to hinder the market growth of industrial hemp.
The market, customers and distribution methods for hemp-based products are large and diverse. These markets range from hemp-based consumables, cosmetics, bio plastics and textiles, to list a few. This is an ever-evolving distribution system that today includes early adopter retailers and ecommerce entities, and product development companies that use our manufacturing capabilities to produce their internally developed consumer products for distribution. In addition, many of our customers use our propriety products and sell them under their own labels.
| 3 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
There are only a few outlets, approximately 60, in mainstream commercial and retail stores that currently stock and sell our products, with the most significant concentration in Arkansas, Tennessee and Texas. However, we believe that as awareness continues to grow for hemp-based products, such as CBD and other products derived from hemp, the market has and will continue to grow over the next several years.
Our target customers are first and foremost end consumers via internet sales, direct-to-consumer retail stores, cooperatives, affiliate sales and master distributors. Secondarily, we are targeting developers of products that we can easily produce with our manufacturing capabilities, national and regional broker networks and major distribution companies who have preexisting relationships with major retail chain stores. As we continue to develop our business, these markets may change, be re-prioritized or eliminated as management responds to consumer and regulatory developments.
Our Competitive Strengths
We attribute our success to the following Growth in CBD Manufacturing.
Growing Market Participant in CBD Product Manufacturing. We are a growing North American distributor and manufacturer of premium CBD products for many of the largest CBD distributors and brands. We manufacture most of our products in our Henderson Nevada leased facility. We believe that loyalty to our brands continues to strengthen as we continue to expand our capabilities and product offering to existing and new customers.
Market Knowledge and Understanding. Due to our experience and our research and development of quality CBD products as well as expansion into new and varied formulations and product categories, we believe our long-term industry relationships will continue to expand. We continue to have a keen understanding of customer needs and desires in both our B2B and B2C customer categories. Custom formulations and a continued commitment to new and improved products at the best possible price has created strong customer demand and a robust pipeline.
Comprehensive Product Offering. We believe we offer the industry’s most comprehensive portfolio of CBD products and maintain over 1,000 SKUs for our customers to choose from. This broad product offering creates a “one-stop” shop for our customers and positively distinguishes us from our competitors. In addition, we are cultivating a portfolio of well-known brands and premium products.
Trade Show Market. Our leading market position in the CBD industry trade show continues to drive sales and market exposure. Although COVID-19 led to cancelation of our 2020 show, we believe that the latest break-throughs with the vaccine and additional precautionary measures will enable us to conduct our next show in the late 2021 expected to take pace in Las Vegas. The brand loyalty and the exposure our show customers receive with premium booth placements has driven a large demand and we anticipate to continue the growth of the tradeshow business in 2022.
Professionalism and Entrepreneurial Culture. Our professionalism and entrepreneurial culture fosters highly-dedicated employees who provide our customers with unsurpassed services. We continue to invest in our talent by providing every sales representative with an extensive and ongoing education and have successfully developed programs that provide comprehensive product knowledge and the tools needed to have a unique understanding of our customers’ personalities and decision-making processes.
Relationships and Superior Service first. We aim to be the premier partner for our customers and suppliers.
|
|
· |
Customers. We strive to offer unsurpassed services and solutions to our customers and also provide comprehensive product offering, proprietary industry formulations and development. We deliver products to our customers in a precise, safe and timely manner with complementary support from our dedicated sales and service teams. |
|
|
|
|
|
|
· |
Suppliers. Our industry knowledge, market reach and resources allow us to establish trusted professional relationships with many of our product suppliers. Our expanding product lines continue to drive demand for our raw materials, the continuing increases have allowed us to negotiate what we believe to be the best possible pricing for our customers, while maintaining a quality growing relationship with the suppliers. |
| 4 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Experienced and Proven Management Team Driving Growth through Organic and Accretive Acquisition Opportunities. We believe our management team has extensive experience in the industry. Our senior management team brings experience in accounting, mergers and acquisitions, financial services, consumer packaged goods, retail operations and third-party logistics.
Our Growth Strategy
We intend to focus or growth on the vertical integration and growth of all segments of the CBD space:
Dependable White/Private Label Manufacturing Service. Our experience and dedicated team continue to refine and expand our white label services, as we are becoming a leading manufacturer for many regional and nationwide brands. Our operations in this segment have doubled over 2020, which we attribute to our commitment to high quality and on time manufacturing services.
Leader in CBD Product Research and Development. Our team provides custom products and proprietary formulations for some of the most popular industry items. We also continue to expand product offerings with the development and launch of new items on a regular basis. Custom formulations for outside brands build long term commitments from our customers.
Direct-to-Consumer Expansion. Our direct-to-consumer business is expected to be our growth driver for the next several years. The lower cost of our in-house research, development and manufacturing give us a measurable cost and production advantage, which we believe to be the key to our future success, as margins in the industry compress and are expected to continue to compress over the next several years.
CBD.io Market Place and Trade Show. Our launch of the CBD.io market platform in 2021 is expected to be a driver for growth into 2022 and a driver of retention for the brands that manufacture for us and list acceptable products on the platform. This high margin business should be a driver for future growth in all segments of the business.
Our leading market position in the CBD industry trade show continues to drive sales and market exposure. Although Covid-19 led to cancelation of our 2020 show, we believe that the latest break-throughs with the vaccine and additional safety measures will enable us to conduct our next show in late 2021 expected to take pace in Las Vegas. The brand loyalty and the exposure our show customers receive with premium booth placements has driven a large demand and we anticipate expansion of shows and venues in 2022.
Core Brand Distribution. The nationwide rollout of our in-house brands will be another substantial driver of growth for the foreseeable future. We began expansion of our sales and marketing teams into the beginning of 2021 and will look to add talented people in all segments of the business to push current and future growth opportunities.
Acquisition Strategy. We have completed two acquisitions in the past two year and the consolidation and recognition of the consolidated synergies are almost completed and expected to be completed prior to the end of the fiscal year. We will continue to search for target acquisitions that meet our acquisition criteria and are accretive to our business. Our platform was built from the ground up to promote acquisitions expansion as a driver of substantial growth as the industry matures and margins compress. Our relationships and partners in the trade show and manufacturing business will be a key source for possible candidates. Our criteria will be stringent, and we will look at any and all opportunities that allow us to use our low cost manufacturing to drive higher margins in acquisition candidates. Small regional brands with distribution would benefit greatly in both low-cost manufacturing and quality research and development of new and current product offerings available from our inhouse brands and products. As margins compress in the industry with the expansion of competition, the low-cost manufacturing capabilities will be a key component to higher profits leading to consolidation which we intend to capitalize on in the coming years.
| 5 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Competition
There is vigorous competition within each market where our CBD products are sold. Brand recognition, quality, performance, availability, and price are some of the factors that impact consumers’ choices among competing products and brands. Advertising, promotion, merchandising and the pace and timing of new product introductions also have a significant impact on consumers’ buying decisions. We compete against several national and international companies, most of which have substantially greater resources than we do. Our principal competitors consist of large, well-known, multinational manufacturers and marketers of CBD products, most of which market and sell their products under multiple brand names. They include, among others, 3CHI, Spring Creek Labs, Kazmira LLC, Global Cannabinoids, Triangle Trading Company, Harbor City Hemp and many others. We also face competition from several independent brands, as well as some retailers that have developed their own CBD brands. Certain of our competitors also have ownership interests in retailers that are customers of ours. While we expect we will seek to address the aspirations of our customers at attainable price points which we believe may give us a competitive advantage, there are no assurances we will ever be able to effectively compete within this sector.
Recent Transactions
Acquisition of HAVZ Consolidated
On May 31, 2019, the Company purchased Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, and One Hit Wonder Holdings, LLC, a California limited liability company, collectively known as “HAVZ Consolidated” out of bankruptcy. Only the one-month period of HAVZ Consolidated was included in the financial statements accompanying this prospectus.
In December of 2018, HAVZ Consolidated filed voluntary petitions for relief under Chapter 11 (Chapter 11 Proceedings) of the U.S. Bankruptcy Code in the U.S. Bankruptcy Court for the District of Nevada. On May 31, 2019, in connection with a sale under Section 363 s of the U.S. Bankruptcy Code, the Company purchased these four entities, HAVZ Consolidated, for a payment of $2,100,000 to the creditors of HAVZ Consolidated.
Acquisition of Infusionz LLC
On July 1, 2020, the Company entered into an Agreement and Plan of Merger with Infusionz LLC (the “Infusionz Agreement”) with the members of Infusionz LLC (the “Sellers”). Pursuant to the terms of the Infusionz Agreement, on July 1, 2020, the Company acquired 100% of the outstanding membership interests of Infusionz LLC, a Colorado limited liability company (“Infusionz”).
Infusionz was formed in the state of Colorado in May 2016. Infusionz develops, manufactures and markets products based on Hemp-based CBD including, but not limited to edibles, tinctures, topicals, capsules and pet products. Infusionz will continue to sell Infusionz branded CBD products for other businesses under their brand and specifications and add the Grove, Inc products, with the expectation of consolidating manufacturing in the Henderson Nevada facility.
Under the purchase method of accounting, the transaction was valued for accounting purposes at an estimated price of $3,350,000, which was the estimated fair value of the consideration paid by the Company. The estimate was based on the consideration paid or payable of $3,000,000 of Common Stock valued and estimated and cash of approximately $350,000, paid based on terms of the Infusionz Agreement. The Company will issue a minimum of 1,500,000 shares of Common Stock to the Sellers. At the close of the acquisition, the Company issued 400,000 shares of Common Stock, valued at the most recent issued price per common share of $0.85 per share. Based on this valuation of the stock value, the Company will issue an additional 3,129,400 shares of Common Stock to the Sellers. The shares of common share will be adjusted based on the common share initial public offering price, as per the Infusionz Agreement. The Company also issued 150,000 shares of Common Stock and recorded an acquisition cost of $127,500 as a finder’s fee.
| 6 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The assets and liabilities of Infusionz will be recorded at their respective fair values as of the closing date of the Infusionz Agreement, and the following table summarizes these values based on the estimated balance sheet at July 1, 2020, the effective closing date.
|
Assets Purchased |
|
$ | 778,331 |
|
|
Liabilities Assumed |
|
|
(680,480 | ) |
|
Net Assets Purchased |
|
|
97,851 |
|
|
Purchase Price |
|
|
(3,350,000 | ) |
|
Intangible Assets and Goodwill from Purchase |
|
$ | 3,402,149 |
|
Summary of Risks Related to Our Business
Our business and our ability to execute our business strategy are subject to a number of risks and unknown factors, as more fully described in the section titled “Risk Factors” beginning on page 13. These risk factors include, among others:
|
|
· |
Our ability to compete and succeed in a highly competitive and evolving industry; |
|
|
· |
Our ability to respond to changing consumer preferences and demand for new products and services; |
|
|
· |
Our ability to identify strategic acquisitions and investments, and to acquire and integrate businesses, product lines and other assets into our Company; |
|
|
· |
Our ability to protect our intellectual property and to develop, maintain and enhance our branded products; |
|
|
· |
The long-term success of our branded consumer products will require significant capital resources and ongoing market adoption of our diverse consumer brands; |
|
|
· |
Our current reliance on certain third parties to conduct various aspects of our vertical business model; |
|
|
· |
Successful launch of the Company’s specialty brands and our industry trade show market platform; |
|
|
· |
The success of our ongoing development, implementation, and optimization of various customer acquisition funnels and our industry trade show market platform; |
|
|
· |
Our ability to achieve and maintain brand loyalty; |
|
|
· |
Our ability to attract and retain highly qualified key employees; |
|
|
· |
Our ability to raise capital and the availability of future financing; |
|
|
· |
Unpredictable events, such as the COVID-19 pandemic, and associated business disruptions which could seriously harm our revenues and financial condition, delay our operations, increase our costs and expenses, and impact our ability to raise capital; |
|
|
· |
The regulatory environment and market acceptance of our diversely branded products and distribution methods; and |
|
|
· |
Regulatory risks and changes in applicable laws, regulations and guidelines. |
Our financial statements have been prepared assuming we will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Our future viability is largely dependent upon our ability to generate profitable operations in the future and to obtain necessary financing as we continue to grow our business. Our management expects that future sources of funding may include sales of equity, obtaining loans, or other strategic transactions. Although our management continues to pursue these plans, there is no assurance that we will be successful with this offering or in obtaining sufficient financing on terms acceptable to us to continue to finance our operations, if at all. These circumstances raise substantial doubt about our ability to continue as a going concern, and our financial statements do not include any adjustments that might result from the outcome of these uncertainties.
| 7 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Implications of Being an Emerging Growth Company and Smaller Reporting Company
We are an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (which we refer to as the “JOBS Act”). As such, we are eligible to take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to SEC reporting companies. For so long as we remain an emerging growth company we will not be required to, among other things:
|
|
· |
present more than two years of audited financial statements and two years of related selected financial data and management’s discussion and analysis of financial condition and results of operations disclosure in our registration statement of which this prospectus forms a part; |
|
|
|
|
|
|
· |
have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002 (which we refer to as the “Sarbanes-Oxley Act”); |
|
|
|
|
|
|
· |
disclose certain executive compensation related items, as we will be subject to the scaled disclosure requirements of a smaller reporting company with respect to executive compensation disclosure; and |
|
|
|
|
|
|
· |
seek stockholder non-binding advisory votes on certain executive compensation matters and golden parachute arrangements. |
We have elected to adopt the reduced disclosure requirements available to emerging growth companies. As a result of these elections, the information that we provide in this prospectus may be different than the information you may receive from other public companies in which you hold equity interests. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the prices of our securities may be more volatile.
The JOBS Act also permits emerging growth companies to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result, our financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an emerging growth company until the earlier of (i) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Common Stock that are held by non-affiliates exceeds $700 million as of the prior the second quarter ending December 31st, and (ii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Finally, we are a “smaller reporting company” (and may continue to qualify as such even after we no longer qualify as an emerging growth company) and accordingly may provide less public disclosure than larger public companies, including the inclusion of only two years of audited financial statements and only two years of management’s discussion and analysis of financial condition and results of operations disclosure. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
Corporate Information
Grove, Inc. was incorporated in the State of Nevada on September 5, 2018. Our principal executive offices are located at 1710 Whitney Mesa Drive, Henderson, NV 89014, and our telephone number is (701) 353-5425. The address of our website is CBD.io. The information contained in, or that can be accessed through, our website is not incorporated by reference into, and is not a part of, this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
| 8 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
Common Stock offered by us |
2,200,000 shares (or 2,530,000 shares if the underwriters exercise their option to purchase additional shares in full). |
|
|
|
|
Over-Allotment option |
We have granted to the underwriters an option to purchase up to additional shares of our Common Stock from us at the initial public offering price less the underwriting discounts and commissions, for a period of 45 days from the date of this prospectus, to cover over-allotments, if any. |
|
|
|
|
Common Stock to be outstanding after this offering |
14,208,333 shares (or 14,583,333 shares if the underwriters exercise the over-allotment option in full). |
|
|
|
|
Use of Proceeds |
We expect to receive net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, of approximately $_______ (or approximately $_______ if the underwriters exercise in full their option to purchase up to _____________ additional shares of our Common Stock), based on an assumed public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus.
We intend to use the net proceeds from this offering (including the additional net proceeds that we would receive if the underwriters exercise their over-allotment option ) for working capital and general corporate purposes, including acquisitions. See “Use of Proceeds.” |
|
|
|
|
Dividend Policy |
We currently intend to retain our future earnings, if any, to finance the development and expansion of our businesses and, therefore, do not intend to pay cash dividends on our Common Stock for the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in any financing instruments, and such other factors as our board of directors deems relevant in its discretion. See “Dividend Policy.” |
|
|
|
|
Risk Factors |
Investing in our Common Stock involves a high degree of risk. For a discussion of factors, you should carefully consider before making an investment decision, see “Risk Factors” beginning on page 13. |
|
|
|
|
Market for the Common Stock |
There has been no market for our securities. We will apply to Nasdaq Capital Market to have our Common Stock listed. |
|
|
|
|
Proposed [Nasdaq-CM] Symbol |
|
| 9 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The number of shares of our Common Stock to be outstanding immediately after this offering will be 14,208,333, which excludes, as of February 9, 2021:(1)
|
|
• |
Shares issuable upon the exercise of the Underwriters’ Warrants to be issued to the representative of the underwriters in this offering; |
|
|
|
|
|
|
• |
166,667 shares of our Common Stock reserved for the exercise of presently outstanding warrants with a weighted average price of $0.85 per share; |
|
|
|
|
|
|
• |
3,666,667 shares of our Common Stock reserved for future grants under our 2019 equity plan; |
|
|
|
|
|
|
• |
1,611,111 shares of our Common Stock reserved for the issuance upon the exercise of presently outstanding options with a weighted average exercise price of $0.85 per share; |
|
|
|
|
|
|
• |
100,000 shares issuable upon conversion of the Convertible Promissory Note, dated October 3, 2019, issued by Registrant in favor of Jeff M. Bishop; |
|
|
· |
100,000 shares issuable upon conversion of the Convertible Promissory Note, dated October 3, 2019, issued by Registrant in favor of Kyle Dennis; |
|
|
· |
100,000 shares issuable upon conversion of the Convertible Promissory Note, dated October 17, 2019, issued by Registrant in favor of Jason Bond; and |
Except as otherwise indicated, all information in this prospectus assumes:
|
|
· |
no exercise by the underwriters’ over-allotment option to purchase additional shares of our Common Stock, |
|
|
|
|
|
|
· |
an assumed offering price per share of $5.00, which is the midpoint of the price range for the shares set forth on the cover page of this prospectus; |
_________
(1) Post-reverse stock split figures
| 10 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL DATA
The following sets forth a summary of our selected consolidated financial and operating information on a historical basis. You should read the following summary of selected consolidated financial information in conjunction with our historical consolidated financial statements, and the related notes thereto, and with the sections entitled “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which are included elsewhere in this prospectus.
Our summary selected historical consolidated statement of operations information for the years ended June 30, 2020 and 2019, unaudited statement of operations for the quarter ended September 30, 2020 and 2019 and our related summary selected historical consolidated balance sheet information as of June 30, 2020 and 2019, summary selected historical consolidated balance sheet information as of September 30, 2020 and 2019 have been derived from our historical audited consolidated financial statements as of and for the years ended June 30, 2020 and 2019, and historical reviewed consolidated financial statements for the quarter ended September 30, 2020 and 2019 which are included elsewhere in this prospectus.
|
|
|
Three Months Ended (unaudited) |
|
|
Year Ended |
| ||||||||||
|
|
|
September 30, |
|
|
June 30, |
| ||||||||||
|
Consolidated Statement of Operations (In U.S. Dollars, except share data or otherwise stated) |
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Product revenue |
|
$ | 2,937,442 |
|
|
$ | 1,669,163 |
|
|
$ | 6,159,012 |
|
|
$ | 1,428,302 |
|
|
Trade show revenue |
|
|
- |
|
|
|
- |
|
|
|
1,253,847 |
|
|
|
779,750 |
|
|
|
|
|
2,937,442 |
|
|
|
1,669,163 |
|
|
|
7,412,860 |
|
|
|
2,208,052 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
1,619,208 |
|
|
|
982,292 |
|
|
|
4,280,909 |
|
|
|
945,756 |
|
|
Trade show costs |
|
|
- |
|
|
|
- |
|
|
|
561,988 |
|
|
|
226,099 |
|
|
|
|
|
1,619,208 |
|
|
|
982,292 |
|
|
|
4,842,897 |
|
|
|
1,171,855 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
1,318,234 |
|
|
|
686,871 |
|
|
|
2,569,963 |
|
|
|
1,036,197 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
365,258 |
|
|
|
304,880 |
|
|
|
1,370,964 |
|
|
|
162,066 |
|
|
General and administrative expenses |
|
|
1,583,641 |
|
|
|
1,237,958 |
|
|
|
5,272,997 |
|
|
|
1,227,361 |
|
|
Professional fees |
|
|
129,421 |
|
|
|
78,012 |
|
|
|
764,332 |
|
|
|
235,823 |
|
|
|
|
|
2,078,320 |
|
|
|
1,620,850 |
|
|
|
7,408,293 |
|
|
|
1,625,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(760,086 | ) |
|
|
(933,979 | ) |
|
|
(4,838,330 | ) |
|
|
(589,053 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other expense (income) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other expense (income), net |
|
|
6,296 |
|
|
|
- |
|
|
|
(180,211 | ) |
|
|
1,055 |
|
|
Impairment of cancelled lease expense |
|
|
- |
|
|
|
- |
|
|
|
588,347 |
|
|
|
- |
|
|
Interest expense (income), net |
|
|
42,691 |
|
|
|
(625 | ) |
|
|
138,406 |
|
|
|
(3,068 | ) |
|
|
|
|
48,987 |
|
|
|
(625 | ) |
|
|
546,542 |
|
|
|
(2,013 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
(809,073 | ) |
|
|
(933,354 | ) |
|
|
(5,384,872 | ) |
|
|
(587,040 | ) |
|
Net income (loss) attributable to noncontrolling interest |
|
|
- |
|
|
|
- |
|
|
|
(1,199 | ) |
|
|
- |
|
|
Net loss attributable to Grove, Inc. |
|
$ | (809,073 | ) |
|
$ | (933,354 | ) |
|
$ | (5,383,673 | ) |
|
$ | (587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings per share attributable to Grove, Inc. Common Stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
$ | (0.04 | ) |
|
$ | (0.05 | ) |
|
$ | (0.30 | ) |
|
$ | (0.05 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding |
|
|
18,691,991 |
|
|
|
17,563,619 |
|
|
|
18,174,735 |
|
|
|
12,647,631 |
|
| 11 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
(unaudited) September 30, |
|
|
June 30, |
| |||||||
|
Consolidated Balance Sheets |
|
2020 |
|
|
2020 |
|
|
2019 |
| |||
|
|
|
|
|
|
|
|
|
|
| |||
|
ASSETS |
|
|
|
|
|
|
|
|
| |||
|
Current assets |
|
|
|
|
|
|
|
|
| |||
|
Cash |
|
$ | 509,000 |
|
|
$ | 887,517 |
|
|
$ | 3,697,432 |
|
|
Accounts receivable, net |
|
|
474,312 |
|
|
|
165,147 |
|
|
|
127,722 |
|
|
Other receivables |
|
|
- |
|
|
|
72,000 |
|
|
|
20,000 |
|
|
Inventory |
|
|
1,893,111 |
|
|
|
1,448,448 |
|
|
|
1,138,064 |
|
|
Prepaid expenses |
|
|
115,283 |
|
|
|
76,562 |
|
|
|
131,976 |
|
|
Total current assets |
|
|
2,991,706 |
|
|
|
2,649,674 |
|
|
|
5,115,194 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and Equipment, net |
|
|
1,649,194 |
|
|
|
1,687,273 |
|
|
|
262,015 |
|
|
Intangible asset, net |
|
|
2,886,261 |
|
|
|
1,240,260 |
|
|
|
1,633,738 |
|
|
Goodwill |
|
|
2,039,371 |
|
|
|
493,095 |
|
|
|
493,095 |
|
|
Other assets |
|
|
37,068 |
|
|
|
37,068 |
|
|
|
- |
|
|
Right-of-use asset |
|
|
327,848 |
|
|
|
294,835 |
|
|
|
- |
|
|
Total other assets |
|
|
6,939,742 |
|
|
|
3,752,531 |
|
|
|
2,388,848 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ | 9,931,448 |
|
|
$ | 6,402,205 |
|
|
$ | 7,504,042 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ | 566,197 |
|
|
$ | 484,333 |
|
|
$ | 271,062 |
|
|
Accrued compensation |
|
|
260,727 |
|
|
|
195,399 |
|
|
|
94,241 |
|
|
Deferred revenue |
|
|
786,264 |
|
|
|
473,320 |
|
|
|
255,633 |
|
|
Accrued liabilities related to acquisition |
|
|
2,960,000 |
|
|
|
- |
|
|
|
287,528 |
|
|
Accrued liabilities |
|
|
308,260 |
|
|
|
221,664 |
|
|
|
199,095 |
|
|
Current portion of notes payable |
|
|
250,612 |
|
|
|
183,595 |
|
|
|
- |
|
|
Convertible note payable |
|
|
1,500,000 |
|
|
|
1,500,000 |
|
|
|
- |
|
|
Current portion of operating lease payable |
|
|
540,657 |
|
|
|
461,123 |
|
|
|
- |
|
|
Total current liabilities |
|
|
7,172,717 |
|
|
|
3,519,434 |
|
|
|
1,107,559 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes payable, net of current portion |
|
|
595,433 |
|
|
|
365,350 |
|
|
|
- |
|
|
Operating lease payable, net of current portion |
|
|
232,297 |
|
|
|
338,040 |
|
|
|
- |
|
|
Total long-term liabilities |
|
|
827,730 |
|
|
|
703,390 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock |
|
|
- |
|
|
|
- |
|
|
|
500 |
|
|
Common Stock |
|
|
21,250 |
|
|
|
18,400 |
|
|
|
17,376 |
|
|
Additional paid in capital |
|
|
9,817,808 |
|
|
|
7,306,164 |
|
|
|
6,438,918 |
|
|
Accumulated deficit |
|
|
(7,908,057 | ) |
|
|
(7,098,984 | ) |
|
|
(1,715,311 | ) |
|
Total stockholders’ equity |
|
|
1,931,001 |
|
|
|
225,580 |
|
|
|
4,741,483 |
|
|
Non-controlling interest in subsidiary |
|
|
- |
|
|
|
1,953,801 |
|
|
|
1,655,000 |
|
|
Total equity |
|
|
1,931,001 |
|
|
|
2,179,381 |
|
|
|
6,396,483 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity |
|
$ | 9,931,448 |
|
|
$ | 6,402,205 |
|
|
$ | 7,504,042 |
|
| 12 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
RISK FACTORS
Investing in our shares of Common Stock is very risky. Before making an investment decision, you should carefully consider all of the risks described in this prospectus. If any of the risks discussed in this prospectus actually occur, our business, financial condition and results of operations could be materially and adversely affected, the price of our shares could decline significantly, and you might lose all or a part of your investment. The risk factors described below are not the only ones that may affect us. Our forward-looking statements in this prospectus are also subject to the following risks and uncertainties. In deciding whether to purchase our shares, you should carefully consider the following factors, among others, as well as information contained in this prospectus.
Risks Relating to Our Company
Our limited operating history makes it difficult for potential investors to evaluate our business prospects and management.
The Company was incorporated on September 5, 2018 and only commenced operations thereafter. Accordingly, we have a limited operating history upon which to base an evaluation of our business and prospects. Operating results for future periods are subject to numerous uncertainties, and we cannot assure you that the Company will achieve or sustain profitability in the future.
The Company’s prospects must be considered in light of the risks encountered by companies in the early stage of development, particularly companies in new and rapidly evolving markets. Future operating results will depend upon many factors, including our success in attracting and retaining motivated and qualified personnel, our ability to establish short term credit lines or obtain financing from other sources, such as this Offering, our ability to develop and market new products, our ability to control costs, and general economic conditions. We cannot assure you that the Company will successfully address any of these risks. There can be no assurance that our efforts will be successful or that we will ultimately be able to attain profitability.
Our business has posted net operating losses since inception in 2018
We incurred losses of $5,383,673 and $587,040 for the years ended June 30, 2020 and 2019, respectively. We have incurred losses of $6,192,746 from inception through September 30,2020. The adverse effects of a limited operating history include reduced management visibility into forward sales, marketing costs, and customer acquisition, which could lead to missing targets for achievement of profitability.
We need additional capital to continue operations; if we do not raise additional capital, we will need to curtail or cease operations.
We require additional capital for the development of our business operations. We may also encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may increase our capital needs and/or cause us to spend our cash resources faster than we expect. Accordingly, we will need to obtain substantial additional funding in order to continue our operations. The uncertainties surrounding our ability to fund our operations raise substantial doubt about our ability to continue as a going concern.
Since our inception, we have financed our operations primarily through the sale of our Common Stock. As of September 30, 2020, we had approximately $509,000 in cash. To execute on our business plan successfully, we will need to raise additional money in the future. Additional financing may not be available on favorable terms, or at all. The exact amount of funds raised, if any, will determine how quickly we can reach profitability on our operations.
There are no assurances that future funding will be available on favorable terms, or at all. If additional funding is not obtained, we may need to reduce, defer or cancel product development efforts, our production and marketing operations, or overhead expenditures to the extent necessary. The failure to fund our operating and capital requirements could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to protect our intellectual property rights, our competitive position could be harmed.
| 13 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Our commercial success will depend in part on our ability to obtain and maintain appropriate intellectual property protection in the United States and foreign countries with respect to our proprietary formulations and products. Our ability to successfully implement our business plan depends on our ability to build and maintain brand recognition using trademarks, service marks, trade dress and other intellectual property. We may rely on trade secret, trademark, patent and copyright laws, and confidentiality and other agreements with employees and third parties, all of which offer only limited protection. The steps we have taken and the steps we will take to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights. If our efforts to protect our intellectual property are unsuccessful or inadequate, or if any third party misappropriates or infringes on our intellectual property, the value of our brands may be harmed, which could have a material adverse effect on the Company’s business and prevent our brands from achieving or maintaining market acceptance. Protecting against unauthorized use of our trademarks, patented technology and other intellectual property rights may be expensive, difficult and in some cases not possible. In some cases, it may be difficult or impossible to detect third-party infringement or misappropriation of our intellectual property rights, and proving any such infringement may be even more difficult.
We may not be able to effectively manage growth.
As we continue to grow our business and develop products, we expect to need additional research, development, managerial, operational, sales, marketing, financial, accounting, legal and other resources. The Company expects its growth to place a substantial strain on its managerial, operational and financial resources. The Company cannot assure that it will be able to effectively manage the expansion of its operations, or that its facilities, systems, procedures or controls will be adequate to support its operations. The Company’s inability to manage future growth effectively would have a material adverse effect on its business, financial condition and results of operations.
Our management may not be able to control costs in an effective or timely manner.
The Company’s management has used reasonable efforts to assess, predict and control costs and expenses. However, the Company only has a brief operating history upon which to base those efforts. Implementing our business plan may require more employees, capital equipment, supplies or other expenditure items than management has predicted. Likewise, the cost of compensating employees and consultants or other operating costs may be higher than management’s estimates, which could lead to sustained losses.
We expect our quarterly financial results to fluctuate.
We expect our net sales and operating results to vary significantly from quarter to quarter due to a number of factors, including changes in:
|
|
· |
Demand for our products; |
|
|
· |
Our ability to obtain and retain existing customers or encourage repeat purchases; |
|
|
· |
Our ability to manage our product inventory; |
|
|
· |
General economic conditions, both domestically and in foreign markets; |
|
|
· |
Advertising and other marketing costs; and |
|
|
· |
Costs of creating and expanding product lines. |
As a result of the variability of these and other factors, our operating results in future quarters may be below the expectations of our stockholders.
We are subject to the reporting requirements of U.S. federal securities laws, which can be expensive.
We are subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including compliance with the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited financial statements to stockholders will cause our expenses to be higher than they would be if we had remained privately-held. In addition, it may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures.
| 14 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Cybersecurity breaches of our IT systems could degrade our ability to conduct our business operations and deliver products and services to our customers, delay our ability to recognize revenue, compromise the integrity of our software products, result in significant data losses and the theft of our intellectual property, damage our reputation, expose us to liability to third parties and require us to incur significant additional costs to maintain the security of our networks and data.
We increasingly depend upon our IT systems to conduct virtually all of our business operations, ranging from our internal operations and product development activities to our marketing and sales efforts and communications with our customers and business partners. Computer programmers may attempt to penetrate our network security, or that of our website, and misappropriate our proprietary information or cause interruptions of our service. Because the techniques used by such computer programmers to access or sabotage networks change frequently and may not be recognized until launched against a target, we may be unable to anticipate these techniques. In addition, sophisticated hardware and operating system software and applications that we produce or procure from third parties may contain defects in design or manufacture, including “bugs” and other problems that could unexpectedly interfere with the operation of the system. We have also outsourced a number of our business functions to third-party contractors, including our manufacturers and logistics providers, and our business operations also depend, in part, on the success of our contractors’ own cybersecurity measures. Similarly, we rely upon distributors, resellers and system integrators to sell our products and our sales operations depend, in part, on the reliability of their cybersecurity measures. Additionally, we depend upon our employees to appropriately handle confidential data and deploy our IT resources in safe and secure fashion that does not expose our network systems to security breaches and the loss of data. Accordingly, if our cybersecurity systems and those of our contractors fail to protect against unauthorized access, sophisticated cyberattacks and the mishandling of data by our employees and contractors, our ability to conduct our business effectively could be damaged in a number of ways, including:
We may incur significant costs and require significant management resources to evaluate our internal control over financial reporting as required under Section 404 of the Sarbanes-Oxley Act, and any failure to comply or any adverse result from such evaluation may have an adverse effect on our stock price.
As a smaller reporting company, as defined in Rule 12b-2 under the Exchange Act, we will be required to evaluate our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”) and to include an internal control report beginning with the Annual Report on Form 10-K for the fiscal year ending June 30, 2022.This report must include management’s assessment of the effectiveness of our internal control over financial reporting as of the end of the fiscal year. This report must also include disclosure of any material weaknesses in internal control over financial reporting that we have identified. Failure to comply, or any adverse results from such evaluation could result in a loss of investor confidence in our financial reports and have an adverse effect on the trading price of our equity securities.
The COVID-19 pandemic and the efforts to mitigate its impact may have an adverse effect on our business, liquidity, results of operations, financial condition and price of our securities.
The pandemic involving the novel strain of coronavirus and related respiratory disease (which we refer to as COVID-19) and the measures taken to combat it, have had an adverse effect on our business. Public health authorities and governments at local, national and international levels have announced various measures to respond to this pandemic. Some measures that directly or indirectly impact our business include:
|
|
· |
voluntary or mandatory quarantines; |
|
|
· |
restrictions on travel; and |
|
|
· |
limiting gatherings of people in public places. |
We have undertaken measures in an effort to mitigate the spread of COVID-19 including limiting company travel and in-person meetings. We also have enacted our business continuity plans, including implementing procedures requiring employees working remotely where possible which may make maintaining our normal level of corporate operations, quality controls and internal controls difficult. Notwithstanding these efforts, our results of operations have been adversely impacted by COVID-19 and this may continue.
| 15 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Moreover, the COVID-19 pandemic has previously caused some temporary delays in the delivery of our inventory, although recently we are no longer experiencing such delays. In addition, the travel restrictions imposed as a result of COVID-19 have impacted our ability to visit customer sites to perform services related to our products. Further, the COVID-19 pandemic and mitigation efforts have also adversely affected our customers’ financial condition, resulting in reduced spending for the products we sell.
As events are rapidly changing, we do not know how long the COVID-19 pandemic, or localized outbreaks or recurrences of COVID-19, and the measures that have been introduced to respond to COVID-19 will disrupt our operations or the full extent of that disruption. Further, once we are able to restart normal operations doing so may take time and will involve costs and uncertainty. We also cannot predict how long the effects of COVID-19 and the efforts to contain it will continue to impact our business after the pandemic is under control. Governments could take additional restrictive measures to combat the pandemic that could further impact our business or the economy in the geographies in which we operate. It is also possible that the impact of the pandemic and response on our suppliers, customers and markets will persist for some time after governments ease their restrictions. These measures have negatively impacted, and may continue to impact, our business and financial condition as the responses to control COVID-19 continue.
A prolonged economic downturn, particularly in light of the COVID-19 pandemic, could adversely affect our business.
Uncertain global economic conditions, in particular in light of the COVID-19 pandemic, could adversely affect our business. Negative global and national economic trends, such as decreased consumer and business spending, high unemployment levels and declining consumer and business confidence, pose challenges to our business and could result in declining revenues, profitability and cash flow. Although we continue to devote significant resources to support our brands, unfavorable economic conditions may negatively affect demand for our products.
Increases in costs, disruption of supply or shortage of raw materials could harm our business.
We may experience increases in the cost or a sustained interruption in the supply or shortage of raw materials. Any such an increase or supply interruption could materially negatively impact our business, prospects, financial condition and operating results. We use various raw materials in our business including aluminum. The prices for these raw materials fluctuate depending on market conditions and global demand for these materials and could adversely affect our business and operating results. Substantial increases in the prices for our raw materials increase our operating costs, and could reduce our margins if we cannot recoup the increased costs through increased prices for our products and services.
Our failure to meet the continuing listing requirements of the NASDAQ Capital Market could result in a de-listing of our securities.
If, after this offering, we fail to satisfy the continuing listing requirements of NASDAQ, such as the corporate governance, stockholders equity or minimum closing bid price requirements, NASDAQ may take steps to delist our Common Stock. Such a delisting would likely have a negative effect on the price of our Common Stock and would impair your ability to sell or purchase our Common Stock when you wish to do so. In the event of a delisting, we would likely take actions to restore our compliance with NASDAQ’s listing requirements, but we can provide no assurance that any such action taken by us would allow our Common Stock to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our Common Stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ’s listing requirements.
| 16 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
We will incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could adversely affect our operating results.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting and corporate governance requirements. These requirements include compliance with Section 404 and other provisions of the Sarbanes-Oxley Act, as well as rules implemented by the Securities and Exchange Commission, or SEC, and the NASDAQ. In addition, our management team will also have to adapt to the requirements of being a public company. We expect complying with these rules and regulations will substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly.
The increased costs associated with operating as a public company will decrease our net income or increase our net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. Additionally, if these requirements divert our management’s attention from other business concerns, they could have a material adverse effect on our business, financial condition and operating results.
As a public company, we also expect that it may be more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as our executive officers.
We are eligible to be treated as an “emerging growth company,” as defined in the JOBS Act, and a “smaller reporting company” within the meaning of the Securities Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies or smaller reporting companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data in this prospectus. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our Common Stock held by non-affiliates exceeds $700.0 million as of any December 31st before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, after which, in each case, we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our shares of Common Stock held by non-affiliates exceeds $250 million as of the prior the end of our second fiscal quarter ending December 31st of each year, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our ordinary shares held by non-affiliates exceeds $700 million as of the prior to the end of our second fiscal quarter ending December 31st of each year. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
After we are no longer an “emerging growth company,” we expect to incur additional management time and cost to comply with the more stringent reporting requirements applicable to companies that are deemed accelerated filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. We cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
| 17 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Risks Relating to Our Business and Industry
We operate in a highly competitive environment, and if we are unable to compete with our competitors, our business, financial condition, results of operations, cash flows and prospects could be materially adversely affected.
We operate in a highly competitive environment. Our competition includes all other companies that are in the business of producing or distributing hemp-based products for personal use or consumption. Many of our competitors have greater resources that may enable them to compete more effectively than us in the CBD industry. Some of our competitors have a longer operating history and greater capital resources, facilities and product line diversity, which may enable them to compete more effectively in this market. Our competitors may devote their resources to developing and marketing products that will directly compete with our product lines. The Company expects to face additional competition from existing competitors and new market entrants. If a significant number of new entrants enters the market in the near term, the Company may experience increased competition for market share and may experience downward pricing pressure on the Company’s products as new entrants increase production. Such competition may cause us to encounter difficulties in generating revenues and market share, and in positioning our products in the market. If we are unable to successfully compete with existing companies and new entrants to the market, our lack of competitive advantage will have a negative impact on our business and financial condition.
Unfavorable publicity or consumer perception of our products or similar products developed and distributed by other companies could have a material adverse effect on our reputation, which could result in decreased sales and fluctuations in our business, financial condition and results of operations.
We depend on consumer perception regarding the safety and quality of our products, as well as similar products marketed and distributed by other companies. Consumer perception of hemp-based products can be significantly influenced by adverse publicity in the form of published scientific research, national media attention or other publicity, which may associate consumption of our products or other similar products with adverse effects, or question the benefits and/or effectiveness of our products or similar products. A new product may initially be received favorably, resulting in high sales of that product, but that level of sales may not be sustainable as consumer preferences change over time. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. Unfavorable research or publicity could have a material adverse effect on our ability to generate sales.
Our failure to appropriately and timely respond to changing consumer preferences and demand for new products and services could significantly harm our customer relationships and have a material adverse effect on our business, financial condition and results of operations.
Our business is subject to changing consumer trends and preferences. Our failure to accurately predict or react to these trends could negatively impact consumer opinion of us as a source for the latest products, which in turn could harm our customer relationships and cause us to lose market share. The success of our product offerings depends upon a number of factors, including our ability to:
|
|
· |
Anticipate customer needs; |
|
|
· |
Innovate and develop new products; |
|
|
· |
Successfully introduce new products in a timely manner; |
|
|
· |
Price our products competitively with retail and online competitors; |
|
|
· |
Deliver our products in sufficient volumes and in a timely manner; and |
|
|
· |
Differentiate our product offerings from those of our competitors. |
If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could be rendered obsolete, which could have a material adverse effect on our financial condition and results of operations.
| 18 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Future acquisitions or strategic investments and partnerships could be difficult to identify and integrate with our business, disrupt our business, and adversely affect our financial condition and results of operations.
We may seek to acquire or invest in businesses and product lines that we believe could complement or expand our product offerings, or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable acquisitions, whether or not the acquisitions are completed. Future acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our financial position and results of operations. In addition, if an acquired business or product line fails to meet our expectations, our business, financial condition, and results of operations may be adversely affected.
Failure to successfully integrate acquired businesses and their products and other assets into our Company, or if integrated, failure to further our business strategy, may result in our inability to realize any benefit from such acquisition.
We expect to grow by acquiring relevant businesses, including other cannabis-related businesses. The consummation and integration of any acquired business, product or other assets into our Company may be complex and time consuming and, if such businesses and assets are not successfully integrated, we may not achieve the anticipated benefits, cost-savings or growth opportunities. Furthermore, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated, expose our Company to increased competition or other challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, technology or other asset or arrangement.
The failure to attract and retain key employees could hurt our business.
Our success also depends upon our ability to attract and retain numerous highly qualified employees. The loss of one or more members of our management team or other key employees or consultants could materially harm our business, financial condition, results of operations and prospects. Although the Company’s current management team has extensive business background, their experience is in industries unrelated to our business. Management relies heavily on the experience of its employees, most notably its President who has extensive experience in CBD products. We face competition for personnel and consultants from other companies, universities, public and private research institutions, government entities and other organizations. Our failure to attract and retain skilled management and employees may prevent or delay us from pursuing certain opportunities. If we fail to successfully fill many management roles, fail to fully integrate new members of our management team, lose the services of key personnel, or fail to attract additional qualified personnel, it will be significantly more difficult for us to achieve our growth strategies and success.
We have limited supply sources, and price increases or supply shortages of key raw materials could materially and adversely affect our business, financial condition and results of operations.
Our products are composed of certain key raw materials. If the prices of such raw materials increase significantly, it could result in a significant increase in our product development costs. If raw material prices increase in the future, we may not be able to pass on such price increases to our customers. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our business, financial condition and results of operations.
The Company believes that its continued success will depend upon the availability of raw materials that permit the Company to meet its labeling claims and quality control standards. The supply of our industrial hemp is subject to the same risks normally associated with agricultural production, such as climactic conditions, insect infestations and availability of manual labor or equipment for harvesting. Any significant delay in or disruption of the supply of raw materials could substantially increase the cost of such materials, could require product reformulations, the qualification of new suppliers and repackaging and could result in a substantial reduction or termination by the Company of its sales of certain products, any of which could have a material adverse effect upon the Company. Accordingly, there can be no assurance that the disruption of the Company’s supply sources will not have a material adverse effect on the Company.
| 19 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Loss of key contracts with our suppliers, renegotiation of such agreements on less favorable terms or other actions these third parties may take could harm our business.
Most of our agreements with suppliers of our industrial hemp, including our key supplier contract, are short term. The loss of these agreements, or the renegotiation of these agreements on less favorable economic or other terms, could limit our ability to procure raw material to manufacture our products. This could negatively affect our ability to meet consumer demand for our products. Upon expiration or termination of these agreements, our competitors may be able to secure industrial hemp from our existing suppliers which will put the company at a competitive disadvantage in the market.
There is limited availability of clinical studies.
Although hemp plants have a long history of human consumption, and the Company believes all of its products to be safe when taken as directed by the Company, there is little long-term experience with human consumption of certain of these innovative product ingredients or combinations thereof in concentrated form. Although the Company performs research and/or tests the formulation and production of its products, there is limited clinical data regarding the safety and benefits of ingesting industrial hemp-based products. Any instance of illness or negative side effects of ingesting industrial hemp-based products would have a material adverse effect on our business and operations.
We face substantial risk of product liability claims and potential adverse product publicity.
Like any other retailer, distributor or manufacturer of products that are designed to be ingested, we face an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused loss or injury. In the event we do not have adequate insurance or contractual indemnification, product liability claims could have a material adverse effect on the Company. The Company is not currently a named defendant in any product liability lawsuit; however, other manufacturers and distributors of hemp-based products currently are or have been named as defendants in such lawsuits. The successful assertion or settlement of any uninsured claim, a significant number of insured claims, or a claim exceeding the Company’s insurance coverage could have a material adverse effect on the Company.
We may be unable to attract and retain independent distributors for our products.
As a direct selling company, our revenue depends in part upon the number and productivity of our independent distributors. Like most direct selling companies, we experience high levels of turnover among our independent distributors from year to year, who may terminate their service at any time. Generally, we need to increase the productivity of our independent distributors and/or retain existing independent distributors and attract additional independent distributors to maintain and/or increase product sales. Many factors affect our ability to attract and retain independent distributors, including the following:
|
|
· |
publicity regarding our Company, our products, our distribution channels and our competitors; |
|
|
· |
public perceptions regarding the value and efficacy of our products; |
|
|
· |
ongoing motivation of our independent distributors; |
|
|
· |
government regulations; |
|
|
· |
general economic conditions; |
|
|
· |
our compensation arrangements, training and support for our independent distributors; and |
|
|
· |
competition in the market. |
Our results of operations and financial condition could be materially and adversely affected if our independent distributors are unable to maintain their current levels of productivity, or if we are unable to retain existing distributors and attract new distributors in sufficient numbers to maintain present sales levels and sustain future growth.
| 20 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The loss of key distributors with significant sales networks could have a material adverse effect on our sales and results of operations.
A significant amount of our net sales, in certain markets, is dependent on a few key distributors and their extensive sales networks. The loss or inactivity of one or more of these key distributors who generate a significant amount of our net sales could have a material adverse effect on our business, results of operations and financial condition.
We could incur obligations resulting from the activities of our independent distributors.
We sell our products through a network of independent distributors. Independent distributors are independent contractors who operate their own business separate and apart from the Company. We may not be able to control certain aspects of our distributors’ activities that may impact our business. If local laws and regulations, or the interpretation thereof, change and require us to treat our independent distributors as employees, or if our independent distributors are deemed by local regulatory authorities in one or more of the jurisdictions in which we operate to be our employees rather than independent contractors under existing laws and interpretations, we may be held responsible for a variety of obligations that are imposed upon employers relating to their employees, including employment-related taxes and penalties, which could have a material adverse effect on our financial condition and results of operations. In addition, there is the possibility that some jurisdictions may seek to hold us responsible for false product or earnings-related claims due to the actions of our independent distributors. Liability for any of these issues could have a material adverse effect on our business, financial condition and results of operations.
Our independent distributors’ failure to comply with applicable advertising laws and regulations could adversely affect our financial conditions and results of operations.
The advertisement of our products is subject to extensive regulations in the markets in which we do business. Our independent distributors may fail to comply with such regulations governing the advertising of our products. We cannot ensure that all marketing materials used by our independent distributors comply with applicable regulations, including bans on false or misleading product and earnings-related claims. If our independent distributors fail to comply with applicable regulations, we could be subjected to claims of false advertising, misrepresentation, significant financial penalties, and/or costly mandatory product recalls and relabeling requirements with respect to our products, any of which could have a material adverse effect on our business, reputation, financial condition and results of operations.
We are subject to risks arising from the recent global outbreak of the COVID-19 coronavirus.
The recent outbreak of the COVID-19 coronavirus has spread across the globe and is impacting worldwide economic activity. A pandemic, including COVID-19 or other public health epidemic, poses the risk that we or our employees, suppliers, manufacturers and other partners may be prevented from conducting business activities for an indefinite period of time, including due to the spread of the disease or shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the full impact that COVID-19 could have on our business, the continued spread of COVID-19 could disrupt our clinical trials, supply chain and the manufacture or shipment of our cyclodextrin products, and other related activities, which could have a material adverse effect on our business, financial condition and results of operations. COVID-19 has also had an adverse impact on global economic conditions which could impair our ability to raise capital when needed. While we have not yet experienced any disruptions in our business or other negative consequences relating to COVID-19, the extent to which the COVID-19 pandemic impacts our results will depend on future developments that are highly uncertain and cannot be predicted.
Risks Related to the CBD Industry
Laws and regulations affecting the CBD industry are evolving under the Farm Bill, and changes to applicable regulations may materially affect our future operations in the CBD market.
In conjunction with the enactment of the Hemp Farming Act of 2018 (the “Farm Bill”), the FDA released a statement about the status of CBD as a nutritional supplement, noting that the Farm Bill explicitly preserved the FDA’s authority to regulate products containing cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic Act (the “FDCA”) and Section 351 of the Public Health Service Act. Any difficulties we experience in complying with existing and/or new government regulation could increase our operating costs and adversely impact our results of operations in future periods.
| 21 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
As a result of the Farm Bill’s recent passage, we expect that there will be a constant evolution of laws and regulations affecting the CBD industry which could affect the Company’s plan of operations. Local, state and federal hemp laws and regulations may be broad in scope and subject to changing interpretations. These changes may require us to incur substantial costs associated with legal compliance and may ultimately require us to alter our business plan. Furthermore, violations of these laws, or alleged violations, could disrupt our business and result in a material adverse effect on our operations. We cannot predict the nature of any future laws, regulations, interpretations or applications, and it is possible that regulations may be enacted in the future that will be directly applicable to our planned business.
Changes to state laws pertaining to industrial hemp could slow the use of industrial hemp, which could impact our revenues in future periods. Approximately 40 states have authorized industrial hemp programs pursuant to the Farm Bill. Continued development of the industrial hemp industry will be dependent upon new legislative authorization of industrial hemp at the state level, and further amendment or supplementation of legislation at the federal level. Any number of events or occurrences could slow or halt progress all together in this space. While progress within the industrial hemp industry is currently encouraging, growth is not assured, and while there appears to be ample public support for favorable legislative action, numerous factors may impact or negatively affect the legislative process(es) within the various states where we have business interests.
Unfavorable interpretations of laws governing hemp processing activities could subject us to enforcement or other legal proceedings and limit our business and prospects.
There are no express protections in the United States under applicable federal or state law for possessing or processing hemp biomass derived from lawful hemp not exceeding 0.3% THC on a dry weight basis and intended for use in finished product, but that may temporarily exceed 0.3% THC during the interim processing stages. While it is a common occurrence for hemp biomass to have variance in THC content during interim processing stages after cultivation but prior to use in finished products, there is risk that state or federal regulators or law enforcement could take the position that such hemp biomass is a Schedule I controlled substance in violation of the CSA and similar state laws. In the event that the Company’s operations are deemed to violate any laws, the Company could be subject to enforcement actions and penalties, and any resulting liability could cause the Company to modify or cease its operations.
Costs associated with compliance with various laws and regulations could impact our financial results.
The manufacture, labeling and distribution of CBD products is regulated by various federal, state and local agencies. These governmental authorities may commence regulatory or legal proceedings, which could restrict our ability to market CBD-based products in the future. The FDA may regulate our products to ensure that the products are not adulterated or misbranded. We may also be subject to regulation by other federal, state and local agencies with respect to our planned CBD-based products. Our advertising activities are subject to regulation by the FTC under the Federal Trade Commission Act. In recent years, the FTC and state attorneys general have initiated numerous investigations of dietary and nutritional supplement companies and products. Any actions or investigations initiated against the Company by governmental authorities or private litigants could have a material adverse effect on our business, financial condition and results of operations. Any actions or investigations initiated against the Company by governmental authorities or private litigants could have a material adverse effect on our business, financial condition and results of operations.
The shifting regulatory environment necessitates building and maintaining of robust systems to achieve and maintain compliance in multiple jurisdictions and increases the possibility that we may violate one or more of the legal requirements applicable to our business and products. If our operations are found to be in violation of any applicable laws or regulations, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, injunctions, or product withdrawals, recalls or seizures, any of which could adversely affect our ability to operate our business, our financial condition and results of operations.
Uncertainty caused by potential changes to legal regulations could impact the use and acceptance of CBD products.
There is substantial uncertainty and differing interpretations and opinions among federal, state and local regulatory agencies, legislators, academics and businesses as to the scope of operation of Farm Bill-compliant hemp programs relative to the emerging regulation of cannabinoids and the Controlled Substances Act. These different opinions include, but are not limited to, the regulation of cannabinoids by the DEA and/or the FDA, and the extent to which manufacturers of products containing Farm Bill-compliant cultivators and processors may engage in interstate commerce. The existing uncertainties in the CBD regulatory landscape in the United States cannot be resolved without further federal, and perhaps state-level, legislation and regulation or a definitive judicial interpretation of existing laws and regulations. If these uncertainties are not resolved in the near future or are resolved in the manner inconsistent with our business plan, such uncertainties may have an adverse effect upon our plan of operations and the introduction of our CBD-based products in different markets.
| 22 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
If we fail to obtain necessary permits, licenses and approvals under applicable laws and regulations, our business and plan of operations may be adversely impacted.
We may be required to obtain and maintain certain permits, licenses and regulatory approvals in the jurisdictions where we sell or plan to sell our products. There can be no assurance that we will be able to obtain or maintain any necessary licenses, permits or approvals. Any material delay in obtaining, or inability to obtain, such licenses, permits and approvals is likely to delay and/or inhibit our ability to carry out our plan of operations, and could have a material adverse effect on our business, financial condition and results of operations.
International expansion of our business could expose us to additional regulatory risks and compliance costs.
If the Company intends to expand internationally or engage in the international sale of its products, it will become subject to the laws and regulations of the foreign jurisdictions in which it operates, or in which it imports or exports products or materials, including, but not limited to, customs regulations in the importing and exporting countries. The varying laws and rapidly changing regulations may impact the Company’s operations and ability to ensure compliance. In addition, the Company may avail itself of proposed legislative changes in certain jurisdictions to expand its product portfolio, which expansion may include unknown business and regulatory compliance risks. Failure by the Company to comply with the evolving regulatory framework in any jurisdiction could have a material adverse effect on the Company’s business, financial condition and results of operations.
The market for CBD products is highly competitive. If we are unable to compete effectively in the market, our business and operating results could be materially and adversely affected.
The market for CBD products is a competitive and rapidly evolving market. There are numerous competitors in the industry, some of whom are more well-established with longer operating histories and greater financial resources than the Company. We expect competition in the CBD industry to continue to intensify following the recent passage of the Farm Bill. We believe the Company will be able to compete effectively because of the quality of our products and customer service. However, there can be no assurance that the Company will effectively compete with existing or future competitors. Increased competition may also drive the prices of our products down, which may have a material adverse effect on our results of operations in future periods.
Given the rapid changes affecting the global, national and regional economies generally, and the CBD industry specifically, the Company may experience difficulties in establishing and maintaining a competitive advantage in the marketplace. The Company’s success will depend on our ability to keep pace with any changes in such markets, especially legal and regulatory changes. Our success will depend on our ability to respond to, among other things, changes in the economy, market conditions and competitive pressures. Any failure to anticipate or respond adequately to such changes could have a material adverse effect on the Company’s business, financial condition and results of operations.
The Company may experience difficulty opening or maintaining bank accounts.
It is possible that financial institutions may refuse to open bank accounts for the deposit of funds from our business, as some of our products are involved with the hemp industry. The inability to open bank accounts with certain institutions could materially and adversely affect our business.
| 23 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Risks Related to this Offering and our Common Stock
Our controlling stockholders may take actions that conflict with your interests.
Certain of our officers and directors beneficially own 55% of our outstanding Common Stock as of the date hereof. Our officers and directors will be able to exercise control over all matters requiring stockholder approval, including the election of directors, amendment of our certificate of incorporation and approval of significant corporate transactions, and they will have significant control over our management and policies. The directors elected by these stockholders will be able to influence decisions affecting our capital structure significantly.
The issuance of additional stock in connection with financings, acquisitions, investments, our stock incentive plans or otherwise will dilute all other stockholders.
Our certificate of incorporation authorizes us to issue up to 100,000,000 shares of Common Stock and up to 10,000,000 shares of preferred stock with such rights and preferences as may be determined by our board of directors. Subject to compliance with applicable rules and regulations, we may issue our shares of Common Stock or securities convertible into our Common Stock from time to time in connection with a financing, acquisition, investment, or otherwise. We may from time to time issue additional shares of Common Stock at a discount from the then market price of our Common Stock. Any issuance of stock could result in substantial dilution to our existing stockholders and cause the market price of our Common Stock to decline.
Our directors have the right to authorize the issuance of shares of our preferred stock and additional shares of our Common Stock.
Our directors, within the limitations and restrictions contained in our certificate of incorporation and without further action by our stockholders, have the authority to issue shares of preferred stock from time to time in one or more series and to fix the number of shares and the relative rights, conversion rights, voting rights, and terms of redemption, liquidation preferences and any other preferences, special rights and qualifications of any such series. While we have no intention of issuing additional shares of preferred stock at the present time, we continue to seek to raise capital through the sale of our securities and may issue shares of preferred stock in connection with a particular investment. Any issuance of shares of preferred stock could adversely affect the rights of holders of our Common Stock. Should we issue additional shares of our Common Stock at a later time, each investor’s ownership interest in our stock would be proportionally reduced.
Our executive officers, directors, major stockholder and their respective affiliates will continue to exercise significant control over our Company after this Offering, which will limit your ability to influence corporate matters and could delay or prevent a change in control.
Immediately following the completion of this Offering and excluding any shares of Common Stock that they purchase in this Offering, if any, the existing holdings of our executive officers and directors will beneficially own approximately _____% of our outstanding Common Stock. As a result, our executive officers and directors will be able to influence our management and affairs and control the outcome of matters submitted to our stockholders for approval, including the election of directors and any sale, merger, consolidation, or sale of all or substantially all of our assets. These stockholders acquired their shares of Common Stock for substantially less than the price of the shares of Common Stock being acquired in this Offering, and these stockholders may have interests, with respect to their Common Stock, that are different from those of investors in this Offering. In addition, the concentration of voting power among these stockholders might adversely affect the market price of our Common Stock by:
|
● |
delaying, deferring or preventing a change in control of the Company; |
|
● |
impeding a merger, consolidation, takeover or other business combination involving the Company; or |
|
● |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of the Company. |
| 24 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
We have broad discretion in how we use the proceeds of this Offering, and may not use these proceeds effectively, which could affect our results of operations and cause the price of our Common Stock to decline.
We will have considerable discretion in the application of the net proceeds of this Offering. We intend to use the net proceeds from this Offering to fund our business strategy, including without limitation, new and ongoing research and development expenses, offering expenses, working capital and other general corporate purposes, which may include funding for the hiring of additional personnel. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this Offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this Offering in a manner that does not produce income or that loses value.
There is no existing market for our Common Stock, and you cannot be certain that an active trading market or a specific share price will be established.
Prior to this Offering, there has been no public market for the shares of our Common Stock. We cannot predict the extent to which investor interest in our Company will lead to the development of a trading market or how liquid that market might become. The Offering price for our Common Stock has been arbitrarily determined by the Company and may not be indicative of the price that will prevail in any trading market following this Offering, if any. The market price for our Common Stock may decline below the Offering price, and our stock price is likely to be volatile.
If our stock price fluctuates after the Offering, you could lose a significant part of your investment.
The market price of our Common Stock could be subject to wide fluctuations in response to, among other things, the risk factors described in this section, and other factors beyond our control, such as fluctuations in the valuation of companies perceived by investors to be comparable to us. Furthermore, the stock markets have experienced price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions, such as recessions, interest rate changes or international currency fluctuations, may negatively affect the market price of our Common Stock. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
If you purchase our Common Stock in this Offering, you will incur immediate and substantial dilution in the book value of your shares.
You will suffer immediate and substantial dilution in the net tangible book value of the Common Stock you purchase in this Offering. Assuming an offering price of $5.00 per share, and assuming all _________ shares are sold for gross proceeds of $_______, purchasers of Common Stock in this Offering will experience immediate dilution of approximately $______ per share in net tangible book value of the Common Stock. In addition, investors purchasing Common Stock in this Offering will contribute up to ___% of the total amount invested by stockholders in the Company since inception but will only own approximately ___% of the shares of Common Stock outstanding. See “Dilution” on page 28 for a more detailed description of the dilution to new investors in the Offering.
After the completion of this Offering, we may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. If our stock price decreases and we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Certain Provisions of Nevada law may have anti-takeover effects.
Certain provisions of Nevada law applicable to our Company could also delay or make more difficult a merger, tender offer or proxy contest involving our company, including Sections 78.411 through 78.444 of the Nevada Revised Statutes, which prohibit a Nevada corporation from engaging in any business combination with any “interested stockholder” (as defined in the statute) for a period of two years unless certain conditions are met. In addition, our senior management is entitled to certain payments upon a change in control.
We do not intend to pay dividends and there will thus be fewer ways in which you can make a gain on your investment.
We do not intend to pay any dividends for the foreseeable future. To the extent that we may require additional funding currently not provided for in our financing plan, our funding sources may prohibit the declaration of dividends. Because we do not intend to pay dividends, any gain on your investment will need to result from an appreciation in the price of our Common Stock. There will therefore be fewer ways in which you can make a gain on your investment.
| 25 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this prospectus contains certain forward-looking statements. All statements other than statements of historical facts contained or incorporated by reference in this prospectus, including statements regarding our future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “will,” “may,” “future,” “plan,” “intend” and “expect” and similar expressions generally identify forward-looking statements. These forward-looking statements are not guaranteed and are subject to known and unknown risks, uncertainties and assumptions that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. Although we believe that our plans, intentions and expectations reflected in the forward-looking statements are reasonable, we cannot be sure that they will be achieved. Particular uncertainties that could cause our actual results to be materially different than those expressed in our forward-looking statements include: our history of losses; our inability to receive regulatory approval for our products; later discovery of previously unknown problems; reliance on third parties; competition between us and other companies in the industry; delays in the development of products; our ability to raise additional capital; continued services of our executive management team; and statements of assumption underlying any of the foregoing, as well as other factors set forth under the caption ”Risk Factors” on page 13 of this prospectus. All subsequent written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by the foregoing. Except as required by law, we undertake no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
USE OF PROCEEDS
We estimate that the net proceeds to us from our sale of ________ shares of our Common Stock in this offering will be approximately $________ (based on an assumed initial public offering price of $_____ per share, which is the midpoint of the price range set forth on the cover page of this prospectus), after deducting the underwriting discounts and commissions and the estimated offering expenses of approximately $______ payable by us.
The underwriters have an option to purchase up to additional shares of our Common Stock at the public offering price less the underwriting discounts and commissions within 45 days after the date of this prospectus to cover-allotments, if any. Exercise by the underwriters of this option in full would result in additional net proceeds to us of approximately $________.
We intend to use the net proceeds from this offering (including the additional net proceeds that we would receive if the underwriters exercise their option to purchase additional shares) for working capital and general corporate purposes, including acquisitions. While we do not have any current agreements, commitments or understandings for any specific acquisitions in connection with which we intend to use a portion of the net proceeds from this offering, we may in the future use a portion of the net proceeds from this offering for such purposes.
Each $1.00 increase (decrease) in the assumed initial public offering price of $_____ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) the net proceeds to us from this offering by approximately $_______, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions payable by us. We may also increase or decrease the number of shares we are selling in this offering. An increase (decrease) of 1,000,000 shares in the number of shares offered by us in this offering, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $______, assuming the assumed initial public offering price of $_____ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) remains the same, and after deducting the underwriting discounts and commissions payable by us. The as-adjusted information discussed above is illustrative only and will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
| 26 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
DIVIDEND POLICY
We currently intend to retain our future earnings, if any, to finance the development and expansion of our businesses and, therefore, do not intend to pay cash dividends on our Common Stock for the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in any financing instruments, and such other factors as our board of directors deems relevant in its sole discretion. Accordingly, you may need to sell your shares of our Common Stock to realize a return on your investment, and you may not be able to sell your shares at or above the price you paid for them. See “Risk Factors—Risks Related to this Offering and our Common Stock— We do not intend to pay dividends and there will thus be fewer ways in which you can make a gain on your investment.”
CAPITALIZATION
The following table sets forth our capitalization as of September 30, 2020, on an:
|
|
· |
actual basis; and |
|
|
|
|
|
|
· |
as adjusted basis to give effect to: |
|
|
· |
the sale and issuance by us of 2,200,000 shares of our Common Stock in this offering at an assumed initial public offering price of $5.00 per share (which is the midpoint of the price range set forth on the cover page of this prospectus), after the payment of the underwriting discounts and commissions and the estimated offering expenses payable by us, and the application of the net proceeds to us from this offering as described in the section entitled “Use of Proceeds”. |
This table should be read in conjunction with the sections entitled “Use of Proceeds,” “Summary Historical Consolidated Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our historical consolidated financial statements, and the related notes thereto, included elsewhere in this prospectus.
|
|
|
As of September 30, 2020 |
| |||||
|
|
|
Actual |
|
|
Proforma |
| ||
|
|
|
(unaudited) |
|
|
|
| ||
|
Cash and cash equivalents |
|
$ | 509,000 |
|
|
|
10,509,000 |
|
|
Total liabilities |
|
$ | - |
|
|
|
|
|
|
Equity: |
|
|
|
|
|
|
|
|
|
Common Stock |
|
$ | 21,250 |
|
|
|
|
|
|
Additional paid in capital |
|
|
9,817,808 |
|
|
|
|
|
|
Parent company investment |
|
|
|
|
|
|
|
|
|
Accumulated deficit |
|
|
(7,908,057 | ) |
|
|
|
|
|
Total stockholders’ equity |
|
|
1,931,001 |
|
|
|
|
|
|
Total capitalization |
|
$ | - |
|
|
|
|
|
Each increase (decrease) of 250,000 shares of Common Stock to be purchased at the assumed offering price of $5.00 per share would increase or (decrease) additional paid-in capital, total shareholders’ equity (deficit) and total capitalization by approximately $1,150,000, assuming the assumed offering price remains at $5.00 and after deducting estimated underwriters’ discounts and commissions and estimated offering expenses payable by us.
| 27 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
A $1.00 increase (decrease) in the assumed public offering price of $5.00 per share of Common Stock would result in an incremental increase (decrease) in each of our additional paid-in capital, total shareholders’ equity (deficit) and total capitalization on a as adjusted basis by approximately $2,024,000, assuming that the number of shares of our Common Stock sold by us as set forth on the cover page of this prospect remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Unless we indicate otherwise, all information in this capitalization section:(1)
|
|
· |
Assumes no exercise by the underwriters of the over-allotment option; |
|
|
· |
Excludes the exercise of the Underwriters’ Warrants to be issued to the representative of the underwriters in this offering; |
|
|
· |
Excludes 119,048 increase in shares of our common stock for each $0.25 decrease of our offering price below $3.60 per common stock; |
|
|
· |
Excludes 166,667 shares of our common stock reserved for the exercise of presently outstanding warrants with a weighted average price of $0.85 per share; |
|
|
· |
Excludes 1,611,111 shares of our common stock reserved for the issuance upon the exercise of presently outstanding options with a weighted average exercise price of $0.85 per share; and |
|
|
· |
Excludes 3,666,667 shares of our common stock reserved for future grants under our 2019 equity plan. |
|
|
(1) |
Post-reverse stock split figures |
DILUTION
Purchasers of shares of our Common Stock in this offering will experience an immediate and substantial dilution in net tangible book value per share of their shares of Common Stock from the assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus).
The difference between the per share initial public offering price paid by purchasers of our Common Stock in this offering and the pro forma net tangible book value per share of our Common Stock after this offering constitutes the dilution to investors in this offering. Net tangible book value per share is determined by dividing our net tangible book value, which is our total tangible assets less total liabilities, by the number of outstanding shares of our Common Stock.
As of September 30, 2020, our net tangible book value was approximately $ , or approximately $ per share of our Common Stock. After giving effect to (i) the sale by us of shares of our Common Stock in this offering, at an assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus), and (ii) the receipt by us of the net proceeds of this offering, after deduction of the underwriting discounts and commissions and the estimated offering expenses payable by us, our pro forma net tangible book value as of September 30, 2020, would have been $ , or approximately $ per share of our Common Stock, representing an immediate increase in net tangible book value of approximately $ per share of our Common Stock to our existing stockholders, and an immediate dilution in net tangible book value of approximately $ per share of our Common Stock to purchasers in this offering.
The following table illustrates the dilution to purchasers in this offering on a per share basis:
|
Initial offering price per share |
|
|
|
$ |
| |||
|
Net tangible book value per share as of September 30, 2020 |
|
$ |
|
|
| |||
|
Increase in net tangible book value per share attributable to purchasers in this offering |
|
$ |
|
|
|
| ||
|
Pro forma net tangible book value per share immediately after this offering |
|
|
|
|
|
$ |
| |
|
Dilution in net tangible book value per share to purchasers in this offering |
|
|
|
|
|
$ |
| |
| 28 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The pro forma net tangible book value per share immediately after this offering is based on the following:
|
Numerator (in thousands): |
|
|
| |
|
Net tangible book value as of September 30, 2020 |
|
$ |
| |
|
Net proceeds to us from this offering(1) |
|
$ |
| |
|
|
|
|
|
|
|
Total pro forma net tangible book value immediately after this offering |
|
$ |
| |
|
Denominator: |
|
|
|
|
|
Shares of our Common Stock outstanding immediately prior to this offering |
|
|
|
|
|
Shares of our Common Stock being sold by us in this offering |
|
|
|
|
|
|
|
|
|
|
|
Total shares of our Common Stock |
|
|
|
|
___________
(1) Assumes no exercise by the underwriters of their option to purchase additional shares of our Common Stock.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) the pro forma net tangible book value per share immediately after this offering by $ per share, and the dilution in pro forma net tangible book value per share to purchasers in this offering by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions payable by us.
We may also increase or decrease the number of shares we are selling in this offering. An increase (decrease) of 1,000,000 shares in the number of shares offered by us in this offering, as set forth on the cover page of this prospectus, would increase (decrease) the pro forma net tangible book value per share immediately after this offering by $ , and the dilution in pro forma net tangible book value per share to purchasers in this offering by $ , assuming the assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) remains the same, and after deducting the underwriting discounts and commissions payable by us.
The tables and information above assume no exercise by the underwriters of their option to purchase additional shares in this offering. If the underwriters exercise in full their option to purchase additional shares of our Common Stock in this offering, the pro forma net tangible book value per share immediately after this offering would be $ per share, and the dilution in pro forma net tangible book value per share to purchasers in this offering would be $ per share, in each case assuming an assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus), and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The following table sets forth, as of September 30, 2020, on the pro forma basis as described above, the differences between the number of shares of our Common Stock purchased or to be purchased from us, the total consideration paid or to be paid to us, and the average price per share paid or to be paid to us, by existing stockholders and by purchasers in this offering, before deducting the underwriting discounts and commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus).
| 29 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
|
Shares Purchased |
|
|
Total Consideration |
| ||||||||||||||
|
|
|
Number |
|
|
Percent |
|
|
Amount |
|
|
Percent |
|
|
Average Price |
| |||||
|
Existing stockholders. |
|
|
|
|
|
% |
|
$ |
|
|
|
% |
|
$ |
| |||||
|
Purchasers from us in this offering |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
100 | % |
|
$ |
|
|
|
100 | % |
|
$ |
| ||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) the total consideration paid by purchasers from us in this offering, total consideration paid by all stockholders, and average price per share paid by all stockholders by $ , $ , and $ per share, respectively, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions payable by us.
Similarly, an increase (decrease) of 1,000,000 shares in the number of shares offered by us in this offering, as set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by purchasers from us in this offering, total consideration paid by all stockholders, and average price per share paid by all stockholders by $ , $ , and $ per share, respectively, assuming the assumed initial public offering price of $ per share (which is the midpoint of the price range set forth on the cover page of this prospectus) remains the same, and after deducting the underwriting discounts and commissions payable by us.
The table and information above assume no exercise by the underwriters of their option to purchase additional shares in this offering. If the underwriters exercise in full their option to purchase additional shares of our Common Stock in this offering, the number of shares held by purchasers from us in this offering would be increased to shares, or % of the total number of shares of our Common Stock outstanding immediately after this offering, and the percentage of shares held by existing stockholders would be reduced to % of the total number of shares of our Common Stock outstanding immediately after this offering.
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent additional capital is raised through the sale of equity securities or other securities convertible into equity securities, the issuance of these securities could result in further dilution to our stockholders. See “Risk Factors—Risks Related to this Offering and our Common Stock.”
| 30 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following in conjunction with the sections of this prospectus entitled “Cautionary Note Regarding Forward-Looking Statements,” “Risk Factors,” “Summary Historical Consolidated Financial Data,” and “Description of Business,” and the historical consolidated financial statements and the related notes thereto included elsewhere in this prospectus. This discussion contains forward-looking statements reflecting current expectations that involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the section entitled “Risk Factors” and elsewhere in this prospectus.
Overview
The Company’s consolidated financial statements are prepared in accordance with GAAP. The consolidated financial statements include the accounts of all subsidiaries in which the Company holds a controlling financial interest as of the financial statement date.
The consolidated financial statements for the year ended June 30, 2020, the year ended June 30, 2019 and the three months ended September 30, 2019 include the accounts of the Company, Trunano Labs, Inc., a Nevada corporation (for which the Company owns 79.4%), and its wholly-owned subsidiaries; Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, One Hit Wonder Holdings, LLC a California limited liability company, SWCH LLC a Delaware limited liability company; and Cresco Management, LLC, a California limited liability company. All intercompany accounts and transactions have been eliminated in consolidation. As of the date of this report, Trunano Labs, Inc. had no operations.
For the three months ended September 30, 2020 the consolidated financial statements of Grove, Inc. include the accounts of the Company and its wholly-owned subsidiaries; Trunano Labs, Inc., a Nevada corporation, Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, One Hit Wonder Holdings, LLC a California corporation; SWCH LLC, a Delaware limited liability company; Cresco Management LLC, a California limited liability company, and Infusionz LLC, a Colorado limited liability company. All intercompany accounts and transactions have been eliminated as a result of the consolidation. As of the date of this report, Trunano Labs, Inc. had no operations.
On May 31, 2019, the Company purchased Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, and One Hit Wonder Holdings, LLC, a California corporation, collectively known as “HAVZ Consolidated”, out of bankruptcy. Only the one-month period of HAVZ Consolidated was included in the 2019 consolidated financial statements.
On July 1, 2020, the Company purchased Infusionz LLC, a Colorado limited liability company.
On July 1, 2020, the noncontrolling shareholders of the Company’s subsidiary, Trunano Labs, Inc., converted 1,761,261 shares of Trunano Labs, Inc., stock, representing all the outstanding stock by minority interest holders, into 2,300,000 shares of Grove Inc. Common Stock. As of July 1, 2020, Trunano Labs, Inc. is a wholly-owned subsidiary of Grove Inc.
| 31 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Operating Segments
The Company’s financial reporting is organized into two segments: products and trade shows for revenue and cost of revenue. The Company’s internal reporting for product sales is organized into three channels of distribution: Grove, Inc. branded products, manufacturing of products to be sold under customers brands and white label products that are sold under customer brands. These product sales are aggregated and viewed by management as one reportable segment due to their similar economic characteristics, products, production, distribution processes and regulatory environment.
The Company does not allocate or track general and administrative expenses to individual reportable segments.
Key Factors Affecting Operating Results
Cyclicality and Seasonality
The trade show business is naturally seasonal. Although management is in the process of expanding the number of trade shows and conference events, currently the Company only has one trade show, CBD.io, which is held in November each year. Because event revenue is recognized when a particular event is held, the Company experiences fluctuations in quarterly revenue based on the completion of the trade show event. The seasonality of the business is typical of the trade show industry. Due to the COVID-19 virus the 2020 CBD.io trade show was suspended and our growth strategy in this area has been delayed.
Non-GAAP Measures
|
Reconciliation of Non-GAAP Adjusted EBITDA to GAAP Net Income (Net Loss) | ||||||||
|
Quarter Ended September 30, | ||||||||
|
|
|
|
|
|
|
| ||
|
|
|
2020 |
|
|
2019 |
| ||
|
Net income (Net loss) GAAP |
|
$ | (809,073 | ) |
|
$ | (933,354 | ) |
|
Interest expense, net |
|
|
42,691 |
|
|
|
(625 | ) |
|
Depreciation and amortization |
|
|
279,899 |
|
|
|
114,037 |
|
|
Stock compensation |
|
|
220,693 |
|
|
|
93,193 |
|
|
Loss on sale of asset |
|
|
6,296 |
|
|
|
- |
|
|
Non-GAAP adjusted EBITDA |
|
$ | (259,494 | ) |
|
$ | (726,749 | ) |
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of Non-GAAP Adjusted EBITDA to GAAP Net Income (Net Loss) | ||||||||
|
Year Ended June 30, | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2019 |
| ||
|
Net income (Net loss) GAAP |
|
$ | (5,383,673 | ) |
|
$ | (587,040 | ) |
|
Interest expense, net |
|
|
(138,406 | ) |
|
|
3,068 |
|
|
Depreciation and amortization |
|
|
611,346 |
|
|
|
67,568 |
|
|
Stock compensation |
|
|
372,770 |
|
|
|
604,557 |
|
|
Impairment of lease cancellation |
|
|
588,347 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP adjusted EBITDA |
|
$ | (3,949,616 | ) |
|
$ | 88,153 |
|
| 32 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Use of Non-GAAP Financial Measures
The Company discloses and uses the above-mentioned non-GAAP financial measures internally as a supplement to GAAP financial information to evaluate its operating performance, for financial planning purposes, to establish operational goals, for compensation plans, to measure debt service capability, for capital expenditure planning and to determine working capital needs and believes that these are useful financial measures also used by investors. Non-GAAP adjusted EBITDA is defined as GAAP net income or net loss before interest, taxes, depreciation and amortization (EBITDA) adjusted for the non-cash stock compensation and stock option expense, acquisition, integration & restructuring expenses, charges and gains or losses from extinguishment of debt. Non-GAAP EBITDA and non-GAAP adjusted EBITDA are not terms defined by GAAP and, as a result, the Company’s measure of non-GAAP EBITDA and non-GAAP adjusted EBITDA might not be comparable to similarly titled measures used by other companies. Generally, a non-GAAP financial measure is a numerical measure of a company’s performance, financial position, or cash flow that either excludes or includes amounts that are not normally included in the most directly comparable measure calculated and presented in accordance with GAAP. The non-GAAP financial measures discussed above, however, should be considered in addition to, and not as a substitute for, or superior to net income or net loss as reported for GAAP on the Consolidated Statements of Income, cash and cash flows on the Consolidated Statement of Cash Flows or other measures of financial performance prepared in accordance with GAAP, and as reflected on the Company’s financial statements prepared in accordance with GAAP. These non-GAAP financial measures are not a substitute for or presented in lieu of financial measures provided by GAAP and all measures and disclosures of financial information pursuant to GAAP should be read to obtain a comprehensive and thorough understanding of the Company’s financial results. The reconciliations of non-GAAP EBITDA and non-GAAP adjusted EBITDA to GAAP operating income (loss) and/or GAAP net income (net loss) referred to in the highlights or elsewhere are provided in the schedules that are a part of this document.
Key Components of Results of Operations
Results of Operations
Three Months Ended September 30, 2020 Compared to Three Months Ended June 30, 2019
|
|
|
September 30, |
|
|
| |||||||
|
|
|
2020 |
|
|
2019 |
|
|
Change |
| |||
|
Revenue |
|
$ | 2,937,442 |
|
|
$ | 1,669,163 |
|
|
$ | 1,268,279 |
|
|
Cost of revenue |
|
|
1,619,208 |
|
|
|
982,292 |
|
|
|
636,916 |
|
|
Operating expenses |
|
|
2,078,320 |
|
|
|
1,620,850 |
|
|
|
457,470 |
|
|
Other expenses |
|
|
48,987 |
|
|
|
(625 | ) |
|
|
49,612 |
|
|
Net loss |
|
$ | (809,073 | ) |
|
$ | (933,354 | ) |
|
$ | (124,281 | ) |
| 33 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Revenues increased by $1,268,279 or 76% compared with the same period last year. Approximately $938,000 was related to the acquisition of Infusionz LLC on July 1, 2020 and the remaining increase was primarily related to CBD sales in the form of gummies, which has experienced significant growth in the recent months.
Cost of revenue increased by $636,916 or 65% compared with the same period last year. Approximately $460,400 was related to the acquisition of Infusionz LLC on July 1, 2020 and the remaining amount was the increase in revenue.
Operating expenses increased by $457,470 or 47% compared with the same period last year. Approximately $496,500 was related to the acquisition of Infusionz LLC on July 1, 2020. The Company’s management is continuing to control operating expenses while also implementing management growth strategies.
The Company incurred a net loss of $809,073 and $933,354 for the three months ended September 30, 2020 and September 30, 2019, respectively. The decrease in the net loss is primarily related to the increase in gross profit.
Segment Information
The Company provides the following distinctive services: (a) product sales and (b) trade show. These distinct services can be divided into two reportable segments, Product Sales and Trade Show Revenue. Selling, general and administrative expenses are not completely separately allocated among these two segments.
Unallocated Corporate expenses primarily include, certain executive compensation expenses and salaries, certain administrative salaries, corporate legal expenses, stock amortization expenses, consulting expenses, audit fees, corporate rent and facility costs, acquisition, integration and restructuring expenses and interest expense.
|
For the three months ended September 30, 2020: |
|
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 2,937,442 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 2,937,442 |
|
|
Costs |
|
|
1,619,208 |
|
|
|
- |
|
|
|
- |
|
|
|
1,619,208 |
|
|
Gross profit |
|
|
1,318,234 |
|
|
|
- |
|
|
|
- |
|
|
|
1,318,234 |
|
|
Gross margin |
|
45 |
% |
|
0 |
% |
|
0 |
% |
|
45 |
% | ||||
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
365,258 |
|
|
|
- |
|
|
|
- |
|
|
|
365,258 |
|
|
Loss on sale of equipment |
|
|
6,292 |
|
|
|
- |
|
|
|
- |
|
|
|
6,292 |
|
|
Depreciation and amortization |
|
|
279,899 |
|
|
|
- |
|
|
|
- |
|
|
|
279,899 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
- |
|
|
|
1,303,742 |
|
|
|
1,303,742 |
|
|
Professional fees |
|
|
129,421 |
|
|
|
- |
|
|
|
- |
|
|
|
129,421 |
|
|
Other expense |
|
|
- |
|
|
|
- |
|
|
|
4 |
|
|
|
4 |
|
|
Interest expense, net |
|
|
42,691 |
|
|
|
- |
|
|
|
- |
|
|
|
42,691 |
|
|
Operating Expenses |
|
|
823,561 |
|
|
|
- |
|
|
|
1,303,746 |
|
|
|
2,127,307 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 494,669 |
|
|
$ | - |
|
|
$ | (1,303,742 | ) |
|
$ | (809,073 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
474,312 |
|
|
|
- |
|
|
|
- |
|
|
|
474,312 |
|
|
Inventory |
|
|
1,893,111 |
|
|
|
- |
|
|
|
- |
|
|
|
1,893,111 |
|
|
Property and Equipment, net |
|
|
1,649,194 |
|
|
|
- |
|
|
|
- |
|
|
|
1,649,194 |
|
|
Intangible asset, less accumulated amortization |
|
|
2,886,261 |
|
|
|
- |
|
|
|
- |
|
|
|
2,886,261 |
|
|
Goodwill |
|
|
1,889,371 |
|
|
|
- |
|
|
|
- |
|
|
|
1,889,371 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
9,199 |
|
|
|
989,199 |
|
|
Total segment assets |
|
$ | 8,792,249 |
|
|
$ | - |
|
|
$ | 989,199 |
|
|
$ | 9,781,448 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 5,454 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 5,454 |
|
| 34 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
For the three months ended September 30, 2019: |
|
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 1,669,163 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,669,163 |
|
|
Costs |
|
|
982,292 |
|
|
|
- |
|
|
|
- |
|
|
|
982,292 |
|
|
Gross profit |
|
|
686,871 |
|
|
|
0 |
|
|
|
- |
|
|
|
686,871 |
|
|
Gross margin |
|
41% |
|
|
0% |
|
|
0% |
|
|
41% |
| ||||
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
304,880 |
|
|
|
- |
|
|
|
- |
|
|
|
304,880 |
|
|
Depreciation and amortization |
|
|
114,037 |
|
|
|
- |
|
|
|
- |
|
|
|
114,037 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
- |
|
|
|
1,123,921 |
|
|
|
1,123,921 |
|
|
Professional fees |
|
|
78,012 |
|
|
|
- |
|
|
|
- |
|
|
|
78,012 |
|
|
Interest income |
|
|
- |
|
|
|
- |
|
|
|
(625 | ) |
|
|
(625 | ) |
|
Operating Expenses |
|
|
496,929 |
|
|
|
- |
|
|
|
1,123,296 |
|
|
|
1,620,225 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 189,942 |
|
|
$ | - |
|
|
$ | (1,123,296 | ) |
|
$ | (933,354 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
26,478 |
|
|
|
- |
|
|
|
- |
|
|
|
26,478 |
|
|
Inventory |
|
|
1,981,809 |
|
|
|
- |
|
|
|
- |
|
|
|
1,981,809 |
|
|
Property and Equipment, net |
|
|
1,290,415 |
|
|
|
- |
|
|
|
- |
|
|
|
1,290,415 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,534,773 |
|
|
|
- |
|
|
|
- |
|
|
|
1,534,773 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
2,085,930 |
|
|
|
2,085,930 |
|
|
Total segment assets |
|
$ | 5,326,570 |
|
|
$ | - |
|
|
$ | 2,085,930 |
|
|
$ | 7,412,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 1,043,472 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,043,472 |
|
(a) The “other” segment is mainly related to the Company’s general expenses and overhead.
| 35 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Year Ended June 30, 2020 as compared to June 30, 2019:
The following summary of our results of operations should be read in conjunction with our consolidated financial statements for the years ended June 30, 2020 and 2019, which are included herein.
|
|
|
June 30, |
|
|
|
| ||||||
|
|
|
2020 |
|
|
2019 |
|
|
Change |
| |||
|
Revenue |
|
$ | 7,412,860 |
|
|
$ | 2,208,052 |
|
|
$ | 5,204,808 |
|
|
Cost of revenue |
|
|
4,842,897 |
|
|
|
1,171,855 |
|
|
|
3,671,042 |
|
|
Operating Expenses |
|
|
7,408,293 |
|
|
|
1,625,250 |
|
|
|
5,783,043 |
|
|
Net loss |
|
$ | (5,384,872 | ) |
|
$ | (587,040 | ) |
|
$ | 4,797,832 |
|
Revenues increased by $5,204,808 or 236% for the fiscal year ended June 30, 2020 compared with the fiscal year ended June 30, 2019. This increase in revenue is attributable to the acquisition of HAVZ Consolidated and the increased Tradeshow revenue related to the CBD.io annual trade show. This increase from Product Revenue was approximately $4,938,377 and approximately $266,431 from the increased trade show revenue. Product revenue increased approximately $746,338 from the prior year HAVZ Consolidated and Grove Inc. consolidated revenue. This was considerably lower than management expectation due to the interruptions of moving and expanding production, increase of development of products sold and decreased sales from the COVID-19 shutdown. Management expects revenue to increase in the 2021 fiscal year, however, does not expect to have revenue from the annual CBD.io trade show as it has been postponed and management is uncertain when the trade show will be scheduled.
Cost of revenue increased by $3,671,042 or 313% compared with the prior fiscal year. This increase in cost of revenue is attributable to the acquisition of HAVZ Consolidated and the increased costs related to the CBD.io annual trade show. This increase from Product Costs was $3,335,153 and from Tradeshow cost was $335,889. Product Costs for HAVZ Consolidated and Grove Inc. consolidated increased approximately $1,187,173 from the prior year. The gross margin of Product Revenue remained consistent from the prior year. The majority of the gross margin decline was related to the lower gross margin in the CBD.io Tradeshow.
Operating expenses increased by $5,783,043 or 356% compared with the prior fiscal year. The increase from the acquisition of HAVZ Consolidated was approximately $2,902,357. In addition, there were approximate increases of $583,000 of professional and consulting fees, $463,958 of payroll expenses, $353,000 of amortization of intangible assets expense, $257,000 of commission expense, $192,000 of depreciation expense, $141,000 of rent costs, $199,000 of marketing advertising expense, $73,000 bad debt expense, and approximately $1,000,000 of total other general and administrative expenses from running multiple facilities during the year ended June 30, 2020 with minimal increases to revenue. These increases were offset by reduction of expenses of approximately $162,000 of royalty expenses and $232,000 of stock-based compensation. The Company’s management consolidated operations and corrected overspending on operating expenses and continues to control operating expenses while also implementing management growth strategies.
| 36 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The Company incurred a net loss of $5,384,872 and $587,040 for the fiscal years ended June 30, 2020 and 2019, respectively. The decrease in the net loss is primarily related to the items noted above and an increase of $588,000 impairment of canceled lease and $141,000 of non-cash interest expense.
The Company incurred a net loss of $5,384,872 and $587,040 for the fiscal years ended June 30, 2020 and 2019, respectively. The increase in the net loss is primarily related to the items noted above and an increase of $588,000 impairment of canceled lease and $141,000 of non-cash interest expense.
Segment Information
The Company provides the following distinctive services: (a) product sales and (b) trade show. These distinct services can be divided into two reportable segments, Product Sales and Trade Show Revenue. Selling, general and administrative expenses are not completely separately allocated among these two segments.
Unallocated Corporate expenses primarily include, certain executive compensation expenses and salaries, certain administrative salaries, corporate legal expenses, stock amortization expenses, consulting expenses, audit fees, corporate rent and facility costs, acquisition, integration and restructuring expenses and interest expense.
|
For the year ended June 30, 2020: |
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 6,159,013 |
|
|
$ | 1,253,847 |
|
|
$ | - |
|
|
$ | 7,412,860 |
|
|
Costs |
|
|
4,280,909 |
|
|
|
561,988 |
|
|
|
- |
|
|
|
4,842,897 |
|
|
Gross profit |
|
|
1,878,104 |
|
|
|
691,859 |
|
|
|
- |
|
|
|
2,569,963 |
|
|
Gross margin |
|
|
30 | % |
|
|
55 | % |
|
|
|
|
|
|
35 | % |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
1,101,137 |
|
|
|
269,827 |
|
|
|
- |
|
|
,370,964 |
| |
|
Impairment on leased equipment |
|
|
588,347 |
|
|
|
- |
|
|
|
- |
|
|
|
588,347 |
|
|
Gain on sale of equipment |
|
|
(180,211 | ) |
|
|
- |
|
|
|
- |
|
|
|
(180,211 | ) |
|
Depreciation and amortization |
|
|
611,346 |
|
|
|
- |
|
|
|
- |
|
|
|
611,346 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
175,225 |
|
|
|
4,486,426 |
|
|
|
4,661,651 |
|
|
Professional fees |
|
|
764,332 |
|
|
|
- |
|
|
|
- |
|
|
|
764,332 |
|
|
Interest expense, net |
|
|
- |
|
|
|
- |
|
|
|
138,406 |
|
|
|
138,406 |
|
|
Operating Expenses |
|
|
2,884,951 |
|
|
|
445,052 |
|
|
|
4,624,832 |
|
|
|
7,954,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | (799,181 | ) |
|
$ | 39,141 |
|
|
$ | (4,624,832 | ) |
|
$ | (5,384,872 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
165,147 |
|
|
|
- |
|
|
|
- |
|
|
|
165,147 |
|
|
Inventory |
|
|
1,448,448 |
|
|
|
- |
|
|
|
- |
|
|
|
1,448,448 |
|
|
Property and Equipment, net |
|
|
1,687,273 |
|
|
|
- |
|
|
|
- |
|
|
|
1,687,273 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,240,260 |
|
|
|
- |
|
|
|
- |
|
|
|
1,240,260 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
1,367,982 |
|
|
|
1,367,982 |
|
|
Total segment assets |
|
$ | 5,034,223 |
|
|
$ | - |
|
|
$ | 1,367,982 |
|
|
$ | 6,402,205 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 1,929,028 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,929,028 |
|
| 37 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
For the year ended June 30, 2019: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ | 1,428,302 |
|
|
$ | 779,750 |
|
|
$ | - |
|
|
$ | 2,208,052 |
|
|
Costs |
|
|
945,756 |
|
|
|
226,099 |
|
|
|
- |
|
|
|
1,171,855 |
|
|
Gross profit |
|
|
482,546 |
|
|
|
553,651 |
|
|
|
- |
|
|
|
1,036,197 |
|
|
Gross margin |
|
|
34 | % |
|
|
71 | % |
|
|
|
|
|
|
47 | % |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
18,302 |
|
|
|
143,764 |
|
|
|
- |
|
|
|
162,066 |
|
|
Depreciation and amortization |
|
|
67,568 |
|
|
|
- |
|
|
|
- |
|
|
|
67,568 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
265,502 |
|
|
|
894,291 |
|
|
|
1,159,793 |
|
|
Professional fees |
|
|
209,815 |
|
|
|
26,008 |
|
|
|
- |
|
|
|
235,823 |
|
|
Other expense |
|
|
- |
|
|
|
- |
|
|
|
1,055 |
|
|
|
1,055 |
|
|
Interest income |
|
|
- |
|
|
|
- |
|
|
|
(3,068 | ) |
|
|
(3,068 | ) |
|
Operating Expenses |
|
|
295,686 |
|
|
|
435,273 |
|
|
|
892,278 |
|
|
|
1,623,237 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 186,860 |
|
|
$ | 118,378 |
|
|
$ | (892,278 | ) |
|
$ | (587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
127,722 |
|
|
|
- |
|
|
|
- |
|
|
|
127,722 |
|
|
Inventory |
|
|
1,138,064 |
|
|
|
- |
|
|
|
- |
|
|
|
1,138,064 |
|
|
Property and Equipment, net |
|
|
262,015 |
|
|
|
- |
|
|
|
- |
|
|
|
262,015 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,633,738 |
|
|
|
- |
|
|
|
- |
|
|
|
1,633,738 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
3,849,408 |
|
|
|
3,849,408 |
|
|
Total segment assets |
|
$ | ,654,634 |
|
|
$ | - |
|
|
$ | 3,849,408 |
|
|
$ | 7,504,042 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 219,448 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 219,448 |
|
_________
(a) The “other” segment is mainly related to the Company’s general expenses and overhead.
| 38 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Liquidity and Capital Resources
Working Capital
|
|
|
As of September 30, 2020 |
|
|
As of June 30, 2020 |
| ||
|
Current assets |
|
$ | 2,991,706 |
|
|
$ | 2,649,674 |
|
|
Current liabilities |
|
$ | 7,022,717 |
|
|
$ | 3,519,434 |
|
|
Working capital |
|
$ | (4,031,011 | ) |
|
$ | (869,760 |
|
Cash Flows
|
|
|
Three Months Ended September 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Cash flows used in operating activities |
|
$ | (637,185 | ) |
|
$ | (2,013,245 | ) |
|
Cash flows provided by (used in) investing activities |
|
|
270,668 |
|
|
|
(1,043,472 | ) |
|
Cash flows (used in) provided by financing activities |
|
|
(12,000 | ) |
|
|
370,000 |
|
|
Net decrease in cash during period |
|
$ | (378,517 | ) |
|
$ | (2,686,717 | ) |
At September 30, 2020, the Company had cash of $509,000 or a decrease of $378,517 from June 30, 2020. Cash flow used in operating activities was $637,185 for the three months ended September 30, 2020. The decrease of cash used in operating activities is primarily related to the decrease in account receivable and inventory and increased non-cash expenses; depreciation and amortization, and shares issued for services.
Net cash provided by (used in) investing activities for the three months ended September 30, 2020 and September 30, 2019 was $270,668 and ($1,043,472), respectively. For the period ended September 30, 2019 the use of cash was primarily due to the acquisition of equipment and for the period ended September 30, 2020 the cash provided was primarily the acquisition of Infusionz LLC, and offset by the sale of property and equipment.
| 39 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Net cash flows (used in) provided by financing activities for the three months ended September 30, 2020 was ($12,000) compared to $370,000 in the three months ended September 30, 2019. Proceeds from the sale of Common Stock and sale of the non-controlling interest during the three months ended September 30, 2019 for $370,000 was the primary reason for the decrease.
These factors raise the doubts about the going concern. The ability to continue as a going concern is dependent upon the Company generating profitable operations in the future and/or obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. Management intends to finance operating costs over the next twelve months from the date of the issuance of these consolidated financial statements with existing cash on hand and/or the private placement of Common Stock. There is, however, no assurance that the Company will be able to raise any additional capital through any type of offering on terms acceptable to the Company, as existing cash on hand will be insufficient to finance operations over the next twelve months.
In December 2019, a novel strain of coronavirus (COVID-19) surfaced. The spread of COVID-19 around the world in 2020 has caused significant volatility in U.S. and international markets. There is significant uncertainty around the breadth and duration of business disruptions related to COVID-19, as well as its impact on the U.S. and international economies and, as such, as of August 12th the Company has transition its operations to 100% work from home and there has been minimal impact to our internal operations from the transition. The Company is unable to determine if there will be a material future impact to its customers’ operations and ultimately an impact to the Company’s overall revenues.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on its financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to stockholders.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP). The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate these estimates, including those related to bad debts, intangible assets, and litigation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of certain assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions.
We have identified below the accounting policies, related to what we believe are most critical to our business operations and are discussed throughout Management’s Discussion and Analysis of Financial Condition or Plan of Operation where such policies affect our reported and expected financial results.
Use of Estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
| 40 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Significant estimates underlying the Company’s reported financial position and results of operations include the allowance for doubtful accounts, useful lives of property and equipment, impairment of long lived assets, inventory valuation, fair value of stock-based compensation and valuation allowance on deferred tax assets.
Business Combinations -The Company accounts for its business combinations using the acquisition method of accounting. The cost of an acquisition is measured as the aggregate of the acquisition date fair values of the assets transferred and liabilities assumed by the Company to the sellers cash consideration and equity instruments issued. Transaction costs directly attributable to the acquisition are expensed as incurred. The excess of (i) the total costs of acquisition over (ii) the fair value of the identifiable net assets of the acquiree is recorded as identifiable intangible assets and goodwill.
Revenue Recognition - The Company has adopted the new revenue recognition guidelines in accordance with ASC 606, Revenue from Contracts with Customers (ASC 606), commencing from the period under this report. The Company analyzes its contracts to assess that they are within the scope and in accordance with ASC 606. In determining the appropriate amount of revenue to be recognized as the Company fulfills its obligations under each of its agreements, whether for goods and services or licensing, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. The Company acts as a principal in its revenue transactions as the Company is the primary obligor in the transactions. Generally, the Company recognizes revenue for its products upon shipment to customers, provided no significant obligations remain and collection is probable.
The Company follows the guidance of the Accounting Standards Codification (“ASC”) Topic 606, “Revenue from Contracts with Customers” which is effective as of the annual reporting period beginning after July 1, 2018 using either of two methods: (1) retrospective application of Topic 606 to each prior reporting period presented with the option to elect certain practical expedients as defined within Topic 606 or (2) retrospective application of Topic 606 with the cumulative effect of initially applying Topic 606 recognized at the date of initial application and providing certain additional disclosures as defined per Topic 606. We adopted Topic 606 pursuant to the method (2) and we determined that any cumulative effect for the initial application did not require an adjustment to retained earnings at July 1, 2018.
Product Revenue - Most of the Company’s revenue contracts are from domestic sales and represent a single performance obligation related to the fulfillment of customer orders for the purchase of its products. Net sales reflect the transaction prices for these contracts based on the Company’s selling list price, which is then reduced by estimated costs for trade promotional programs, consumer incentives, and allowances and discounts used to incentivize sales growth and build brand awareness.
The Company recognizes revenue at the point in time that control of the ordered product is transferred to the customer, which is upon shipment to the customer or other customer-designated delivery point. Taxes collected from customers that are remitted to governmental agencies are accounted for on a net basis and not included as revenue.
The Company does not accept sales returns from wholesale customers unless the sales return was pre-approved prior to production and shipment. E-Commerce product returns must be completed within 45 days of the date of purchase. The Company does not accrue for estimated sales returns as historical sales returns have been minimal. The Company records deferred revenues when cash payments are received or due in advance of performance, including amounts which are refundable. Substantially all the deferred revenue as of June 30, 2019 was recognized as revenue in the year ended June 30, 2020.
| 41 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Shipping and handling fees billed to customers are included in revenue. Shipping and handling fees associated with freight are generally included in cost of revenue.
Trade Show Revenue - A significant portion of the Company’s annual revenue is generated from the production of a single trade show. The revenue includes booth space sales, registration fees and sponsorship fees. The Company recognizes revenue upon completion of the CBD.IO trade show. Amounts invoiced prior to the completion of the trade show are recorded as deferred revenue in the consolidated Balance Sheets until the completion of the event. As of June 30, 2020, and 2019, the Company had no deferred revenue related to trade show business.
Impairment of Long-lived Assets - Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the asset may not be recoverable. The Company periodically evaluates whether events and circumstances have occurred that indicate possible impairment. When impairment indicators exist, the Company estimates the future undiscounted net cash flows of the related asset or asset group over the remaining life in measuring whether or not the asset values are recoverable. The Company did not recognize impairment on its long-lived assets during the years ended June 30, 2020 or 2019.
Stock Based Compensation - The Company recognizes all share-based payments to employees, including grants of employee stock options, as compensation expense in the financial statements based on their fair values. That expense will be recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period) or immediately if the share-based payments vest immediately.
| 42 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
DESCRIPTION OF BUSINESS
Our Company
We are in the business of developing, producing, marketing and selling raw materials, white label products and end consumer products containing the industrial hemp plant extract, Cannabidiol (“CBD”). We sell to numerous consumer markets including the nutraceutical, beauty care, pet care and functional food sectors. We seek to take advantage of an emerging worldwide trend to re-energize the production of industrial hemp and to foster its many uses for consumers. The development of products in this highly regulated industry carries significant risks and uncertainties that are beyond our control. As a result, we cannot assure that we will successfully market and sell our planned products or, if we are able to do so, that we can achieve sales volume levels that will allow us to cover our fixed costs.
The Company primarily conducts its business operations through its wholly-owned subsidiaries: Steam Distribution, LLC, a California limited liability company (“Steam Distribution”), One Hit Wonder, Inc., a California corporation (“One Hit Wonder”), Havz, LLC, d/b/a Steam Wholesale, a California limited liability company (“Steam Wholesale”), and One Hit Wonder Holdings, LLC, a California limited liability company (“OHWH”, and collectively known with Steam Distribution, One Hit Wonder, and Steam Wholesale as “HAVZ Consolidated”); SWCH LLC, a Delaware limited liability company (“SWCH”); Trunano Labs, Inc., a Nevada corporation (“Trunano”); Infusionz LLC, a Colorado limited liability company (“Infusionz”); and Cresco Management, LLC, a California limited liability company (“Cresco”).
Historically cultivated for industrial and practical purposes, hemp is used today for textiles, paper, auto parts, biofuel, cosmetics, animal feed, supplements and much more — an impressive scope for such a historically misunderstood and restricted commodity. The market for hemp-derived products is expected to increase exponentially over the next five years, and we believe Grove is well positioned to be a dominant player in the hemp industry.
In the U.S., hemp products are generally regulated by the Agriculture Improvement Act of 2018 (United States) (the “2018 Farm Bill”). Consequently, the Company processes, develops, manufactures, and sells its products pursuant to the 2018 Farm Bill. CBD products produced and sold by Grove Inc. constitute hemp under the 2018 Farm Bill.
In addition, through one of our wholly owned subsidiaries, we produce primarily business-to-business CBD related trade shows in the United States and were looking to expand prior to the COVID-19 pandemic. The trade shows have been profitable and allow Grove to market its own CBD products and services while also increasing the awareness of the expanding CBD market to the public.
The following is the ownership structure chart of the Company and its wholly owned subsidiaries:
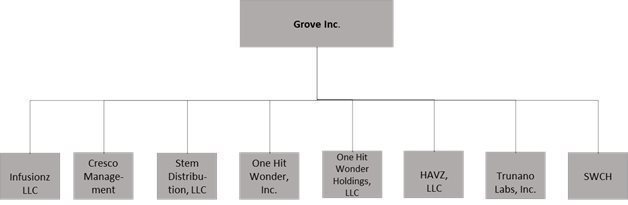
Grove is committed to providing high quality hemp products and services at competitive prices in retail, white label, private label and custom formulation programs. Our white label manufacturing is the partner of choice for many of the industry’s leading brands and the list brands and products we service continues to expand. We have also set out to develop a world-class portfolio of our own proprietary brands that we believe will, over time, deliver higher margins and create long-term value.
We operate manufacturing and distribution centers in Las Vegas, Nevada and Denver, Colorado and expect to expand into the eastern US with a new sale and distribution center in Florida scheduled to be opened in late 2021. We currently export to several European countries and have begun searching for a quality distribution partner familiar with European regulations and distribution processes.
Our History
Grove, Inc. was incorporated in the State of Nevada on September 5, 2018 and has seven wholly-owned subsidiaries: Trunano, Cresco, Steam Distribution, LLC, One Hit Wonder, Inc., Havz, LLC, d/b/a Steam Wholesale, One Hit Wonder Holdings, LLC, SWCH, and Infusionz.
The Company formed Trunano on May 6, 2019 subsequently selling shares in the company to minority interest investors. On July 1, 2020, the noncontrolling shareholders of Trunano converted 1,761,261 shares of common stock of Trunano, representing all the outstanding stock by minority interest holders, into 2,300,000 shares of common stock of the Company, resulting in Trunano becoming a wholly owned subsidiary of the Company. The acquisition of the shares was completed to eliminate the minority interest in Trunano enabling Grove, Inc. management to eliminate any conflicts or related party transactions in future operations. Trunano has no material operations.
| 43 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
On January 1, 2019, all the outstanding membership interests of Cresco were contributed to the Company in exchange for 3,000,000 shares of Grove, Inc. common stock. All operations of Cresco were to support the operations of HAVZ Consolidated.
On January 1, 2019, all the outstanding membership interests of SWCH were contributed to the Company in exchange for 1,700,000 shares of Grove, Inc. common stock. SWCH produced the annual CBD.io tradeshow, a key marketing and brand awareness tool for the Company.
On May 31, 2019, the Company purchased all of the outstanding equity interests of HAVZ Consolidated in a Section 363 Sale from the creditors of HAVZ Consolidated following the filing by HAVZ Consolidated of voluntary petitions for relief in December 2018 under Chapter 11 of the U.S. Bankruptcy Code in the U.S. Bankruptcy Court for the District of Nevada. The Company acquired the entities of HAVZ Consolidated for its manufacturing capabilities, product formulas, customer base and brand recognition.
On July 1, 2020, the Company entered into an Agreement and Plan of Merger with Infusionz. Pursuant to the terms of the agreement, on July 1, 2020, the Company acquired 100% of the outstanding membership interests of Infusionz. The Company acquired Infusionz for its customer base, brand recognition and expansion of CBD product offerings.
Our Products
Grove, Inc. is focused on the manufacturing of CBD products through custom manufacturing, wholesale distribution and retail sales. Our primary products are Gummies, Tinctures, Topical Cosmetics and Flower, with a variety of formulas of cannabinoids and other additives to combat pain, depression, and anxiety and also to provide better calm and sleep to the end user. Our products use Full-spectrum CBD, Broad-spectrum and CBD isolate.
|
|
CBDTincture (full spectrum) GRN CBD Oil Tinctures are filled with USA grown hemp extract. We contract with farms that only produce 100% vegan, pesticide-free, federally legal hemp. Our mixologist then combines our pure CBD oil with MCT oil and other 100% natural ingredients and flavorings to deliver GRN CBD Oils. GRN Full spectrum tinctures include legal levels of trace amounts of THC (<0.3%). |
|
|
|
|
|
CBD Tincture (broad spectrum) GRN CBD Oil Tinctures are filled with USA grown hemp extract. We contract with farms that only produce 100% vegan, pesticide-free, federally legal hemp. Our mixologist then combines our pure CBD oil with MCT oil and other 100% natural ingredients and flavorings to deliver GRN CBD Oils. GRN broad spectrum tinctures contain zero THC while the full spectrum tinctures will include legal levels of trace amounts of THC (<0.3%).
|
| 44 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Oral Strips TruNano Labs, Inc. 15mg CBD Sublingual Breath Strips
|
|
|
|
|
|
CBD Gummies GRN CBD Gummies made from USA grown hemp and contain less than 0.3% THC. ach gummy contains 10mg Full Spectrum CBD in assorted tropical fruit flavors.
|
|
|
|
|
|
CBD Lotion Broad Spectrum Hemp Infused Lotion
|
| 45 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Bath Bombs GRN Broad Spectrum CBD Bath Bombs. Each 6 oz. bath bomb contains 35mg of our hemp extract with your choice of three invigorating aromas: Cedarwood & Tangerine, Lavender & Grapefruit, Eucalyptus & Lemongrass.
|
|
|
|
|
|
CBD Pre-Rolls
|
| 46 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Flower
|
|
|
|
|
|
CBN Capsules CBN Capsules Ingredients: Organic Coconut Flour, Hypromellose (vegetable capsule), Purified Water, Naturally Occurring Hemp CBN, NANO Water Soluble CBD Powder (maltodextrin, MCT, naturally occurring cannabinoids, natural flavors)
|
| 47 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBN Fruit Snacks CBN Fruit Snacks Ingredients: Tapioca Syrup, Organic Cane Sugar, Pear Juice, Pectin, Citric Acid, Sodium Citrate, Malic Acid, Natural Flavors, Natural Colors (black carrot, turmeric), Naturally Occurring Hemp CBN, NANO Water Soluble CBD Powder (maltodextrin, MCT, naturally occurring cannabinoids, natural flavors)
|
|
|
|
|
|
CBG Oil Tinctures CBG Oil Tincture Ingredients: Medium Chain Triglyceride (MCT) Oil (high grade coconut oil), Naturally Occurring CBG, Naturally Occurring CBD
|
|
|
|
|
|
CBD Tonic
|
| 48 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Coffee Ultra premium coffee blend is a medium-bodied coffee with consistent flavor notes of vanilla, caramel, and natural sweetness. Our water soluble CBD coffee consists of 100% Arabica coffee beans grown high in the Colombian mountains.
|
|
|
|
|
|
CBD Drink Mixes Ingredients: Sugar, Dextrose, Citric Acid, Salt, Sodium Citrate, Monopotassium Phosphate, Calcium Silicate, Modified Food Starch, Natural & Artificial Flavor, Yellow5, Hemp CBD Water Soluble Powder (Maltodextrin, MCT, Naturally Occurring Cannabinoids, Natural Flavors)
|
|
|
|
|
|
CBD Lollipops Ingredients: Sugar, Glucose Syrup, Water, Citric Acid, Artificial Flavors (Cherry), Artificial Colors (FD&C Red 40), Titanium Dioxide, Naturally Occurring Cannabinoid Oil
|
| 49 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Chocolate Ingredients: Sugar, Partially Hydrogenated Palm Kernel & Cottonseed Oils, Nonfat Dry Milk, Cocoa (processed with alkali), Cocoa, Glyceryl Lacto Esters of Fatty Acids, Soy Lecithin (emulsifier), Salt, Naturally Occurring Broad Spectrum Cannabinoids CONTAINS: Milk & Soy (Packaged in the same facility as peanuts, tree nuts & wheat) |
|
|
|
|
|
CBD Caramel Waffle Cookies Ingredients: Syrup, (glucose-fructose syrup, sugar syrup) Wheat Flour, Butter (milk), Sugar, Vegetable Oil (palm, rapeseed), Soya Flour, Salt Emulsifier (soya lecithin), Raising Agent (E500), Cinnamon, Natural Bourbon Vanilla, Acidulant (citric acid), Naturally Occurring Cannabinoid Oil |
| 50 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Honey Ingredients: Organic Honey, Full Spectrum Cannabinoid Oil
|
|
|
|
|
|
CBD Muscle Freeze Aloe Gel Ingredients: Menthol USP Natural, Naturally Occurring Cannabinoid Oil, Aloe Vera Extract, Armica Montana Extract, Carbomer, FD&C Yellow 10, Ilex Paraguanensis Leaf Extract, Isopropyl Alcohol Methylparaben, Tea Tree Oil, Tocopheryl Acetate (Vitamin E), Triethanolamine, Water |
| 51 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Massage Oil Ingredients: Purified Water, Isopropyl Palmitate, Propylene Glycol, Glyceryl Sterate, Isononyl Isonanoate, Glycerin, Lanolin Oil, Myristyl Myristate, Steraric Acid, Carbmer, Methylparaben, Diazolidinyl Urea, Iodopropynyl Butylcarbamate, Disodium EDTA, Allation, Triethanolamine, Sorbitan Sterate, Polysorbate, Dimethicone, Propylparaben, BHA Tenox, Aloe Vera Gel, Vitamin A, Vitamin D, Tocopheryl Acetate, Naturally Occurring Cannabinoid Oil |
|
|
|
|
|
CBD Pet Oil
|
| 52 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
CBD Dog Treats Ingredients: Yellow Peas, Dried Bacon Fat, Dried Potatoes, Dried Sweet Potatoes, Canola Oil (Preserved with mixed Tocopherols), Cane Molasses, Organic Carrots, Organic Apples, Cranberries, Blueberries, Rosemary Extract, Naturally Occurring Cannabinoid Oil
|
|
|
|
|
|
CBD Concentrates
|
|
|
|
|
|
CBD E-Liquid Ingredients: Food grade PG, Naturally Occurring Full Spectrum Cannabinoids Naturally Derived Plant Steam Distilled Terpenes
|
| 53 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Market Opportunity
The industrial hemp market is projected to grow at a CAGR of 34% from USD 4.6 billion in 2019 to USD 26.6 billion by 2025. The growth of this market is attributed to the increased consumption of hemp-based products due to their various health benefits and increased incidences of diseases such as epilepsy and other sleep disorders. However, the complex regulatory structure for the usage of industrial hemp in different countries is expected to hinder the market growth of industrial hemp.
The market, customers and distribution methods for hemp-based products are large and diverse. These markets range from hemp-based consumables, cosmetics, bio plastics and textiles, to list a few. This is an ever-evolving distribution system that today includes early adopter retailers and ecommerce entities, and product development companies that use our manufacturing capabilities to produce their internally developed consumer products for distribution. In addition, many of our customers use our propriety products and sell them under their own labels.
There are only a few outlets, approximately 60, in mainstream commercial and retail stores that currently stock and sell our products, with the most significant concentration in Arkansas, Tennessee and Texas. However, we believe that as awareness continues to grow for hemp-based products, such as CBD and other products derived from hemp, the market has and will continue to grow over the next several years.
Our target customers are first and foremost end consumers via internet sales, direct-to-consumer retail stores, cooperatives, affiliate sales and master distributors. Secondarily, we are targeting developers of products that we can easily produce with our manufacturing capabilities, national and regional broker networks and major distribution companies who have preexisting relationships with major retail chain stores. As we continue to develop our business, these markets may change, be re-prioritized or eliminated as management responds to consumer and regulatory developments.
Our Competitive Strengths
We attribute our success to the following Growth in CBD Manufacturing.
Growing Participant Leader in CBD Product Manufacturing. We are a growing North American distributor and manufacturer of premium CBD products for many of the largest CBD distributors and brands. We manufacture most of our products in our Henderson Nevada leased facility. We believe that loyalty to our brands continues to strengthen as we continue to expand our capabilities and product offering to existing and new customers.
Market Knowledge and Understanding. Due to our experience and our research and development of quality CBD products as well as expansion into new and varied formulations and product categories, we believe our long-term industry relationships will continue to expand. We continue to have a keen understanding of customer needs and desires in both our B2B and B2C customer categories. Custom formulations and a continued commitment to new and improved products at the best possible price has created strong customer demand and a robust pipeline.
Comprehensive Product Offering. We believe we offer the industry’s most comprehensive portfolio of CBD products and maintain over 1,000 SKUs for our customers to choose from. This broad product offering creates a “one-stop” shop for our customers and positively distinguishes us from our competitors. In addition, we are cultivating a portfolio of well-known brands and premium products.
Trade Show Market. Our leading market position in the CBD industry trade show continues to drive sales and market exposure. Although COVID-19 led to cancelation of our 2020 show, we believe that the latest break-throughs with the vaccine and additional precautionary measures will enable us to conduct our next show in the late 2021 expected to take pace in Las Vegas. The brand loyalty and the exposure our show customers receive with premium booth placements has driven a large demand and we anticipate continuing the growth of the tradeshow business in 2022.
| 54 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Professionalism and Entrepreneurial Culture. Our professionalism and entrepreneurial culture foster highly-dedicated employees who provide our customers with unsurpassed services. We continue to invest in our talent by providing every sales representative with an extensive and ongoing education and have successfully developed programs that provide comprehensive product knowledge and the tools needed to have a unique understanding of our customers’ personalities and decision-making processes.
Relationships and Superior Service first. We aim to be the premier partner for our customers and suppliers.
|
|
· |
Customers. We strive to offer unsurpassed services and solutions to our customers and also provide comprehensive product offering, proprietary industry formulations and development. We deliver products to our customers in a precise, safe and timely manner with complementary support from our dedicated sales and service teams. |
|
|
|
|
|
|
· |
Suppliers. Our industry knowledge, market reach and resources allow us to establish trusted professional relationships with many of our product suppliers. Our expanding product lines continue to drive demand for our raw materials, the continuing increases have allowed us to negotiate what we believe to be the best possible pricing for our customers, while maintaining a quality growing relationship with the suppliers. |
Experienced and Proven Management Team Driving Growth through Organic and Accretive Acquisition Opportunities. We believe our management team has extensive experience in the industry. Our senior management team brings experience in accounting, mergers and acquisitions, financial services, consumer packaged goods, retail operations and third-party logistics.
Our Growth Strategy
Our growth will continue to be focused on the vertical integration and growth of all segments of the CBD space:
Dependable White/Private Label Manufacturing Service. Our experience and dedicated team continue to refine and expand our white label services and has become a leader to many regional and nationwide brands. Our operations in this segment have doubled over 2020, which we attribute to our commitment to high quality and on time manufacturing services.
Leader in CBD Product Research and Development. Our team provides custom products and proprietary formulations for some of the most popular industry items. We also continue to expand product offerings with the development and launch of new items on a regular basis. Custom formulations for outside brands build long term commitments from our customers.
Direct-to-Consumer Expansion. Our direct-to-consumer business is expected to be our growth driver for the next several years. The lower cost of our in-house research, development and manufacturing give us a measurable cost and production advantage, which we believe to be the key to our future success, as margins in the industry compress and are expected to continue to compress over the next several years.
CBD.io Market Place and Trade Show. Our launch of the CBD.io market platform in 2021 is expected to be a driver for growth into 2022 and a driver of retention for the brands that manufacture for us and list acceptable products on the platform. This high margin business should be a driver for future growth in all segments of the business.
Our leading market position in the CBD industry trade show continues to drive sales and market exposure. Although COVID-19 led to cancelation of our 2020 show, we believe that the latest break-throughs with the vaccine and additional safety measures will enable us to conduct our next show in late 2021 expected to take pace in Las Vegas. The brand loyalty and the exposure our show customers receive with premium booth placements has driven a large demand and we anticipate expansion of shows and venues in 2022.
| 55 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Core Brand Distribution. The nationwide rollout of our in-house brands will be another substantial driver of growth for the foreseeable future, we began expansion of our sales and marketing teams into the beginning of 2021 and will look to add talented people in all segments of the business to push current and future growth opportunities.
Acquisition Strategy. We have completed two acquisitions with the consolidation and synergies are almost completed and expected to be completed prior to June 30, 2021. We will continue to search for target acquisitions that meet our acquisition criteria and are accretive to our business. Our platform was built from the ground up to promote acquisitions expansion as a driver of substantial growth as the industry matures and margins compress. Our relationships and partners in the trade show and manufacturing business will be a key source for possible candidates. Our criteria will be stringent and we will look at any and all opportunities that allow us to use our low cost manufacturing to drive higher margins in acquisition candidates. Small regional brands with distribution would benefit greatly in both low-cost manufacturing and quality research and development of new and current product offerings available from our inhouse brands and products. As margins compress in the industry, the low-cost manufacturing capabilities will be a key component to higher profits leading to consolidation which we intend to capitalize on in the coming years.
Competition
There is vigorous competition within each market where our CBD products are sold. Brand recognition, quality, performance, availability, and price are some of the factors that impact consumers’ choices among competing products and brands. Advertising, promotion, merchandising and the pace and timing of new product introductions also have a significant impact on consumers’ buying decisions. We compete against several national and international companies, most of which have substantially greater resources than we do. Our principal competitors consist of large, well-known, multinational manufacturers and marketers of CBD products, most of which market and sell their products under multiple brand names. They include, among others, 3CHI, Spring Creek Labs, Kazmira LLC, Global Cannabinoids, Triangle Trading Company, Harbor City Hemp and many others. We also face competition from several independent brands, as well as some retailers that have developed their own CBD brands. Certain of our competitors also have ownership interests in retailers that are customers of ours. While we expect we will seek to address the aspirations of our customers at attainable price points which we believe may give us a competitive advantage, there are no assurances we will ever be able to effectively compete within this sector.
Government Regulation
We are subject to laws and regulations affecting our operations in a number of areas. These laws and regulations affect the Company’s activities in areas, including, but not limited to, the hemp business in the United States, the consumer products and nutritional supplement markets in the United States, consumer protection, labor, intellectual property ownership and infringement, import and export requirements, federal and state healthcare, environmental and safety. The successful execution of our business objectives will be contingent upon our compliance with all applicable laws and regulations and obtaining all necessary regulatory approvals, permits and registrations, which may be onerous and expensive. Any such costs, which may rise in the future as a result of changes in such applicable laws and regulations and the expansion of the Company’s business, could make our products and services less attractive to our customers, delay the introduction of new products, and require the Company to implement policies and procedures designed to ensure compliance with applicable laws and regulations.
We operate our business in markets that are both highly regulated and rapidly evolving. We are subject to numerous federal and state laws and regulations affecting the manufacturing, packaging, labeling and sale of food, beverages, dietary supplements, and personal care products/cosmetics, as well as the use of hemp and hemp-derived ingredients like CBD in such products. While there are no specific laws or regulations enforced by the FDA pertaining to the use of hemp and hemp-derived ingredients in FDA-regulated products, the FDA has issued guidance on the subject and issued letters to companies regarding claims made for products and the use of such ingredients in various products. The FDA also initiated a task force to evaluate pathways for further regulation of hemp and hemp-derived ingredients. At various times, bills pertaining to the regulation of hemp and hemp-derived ingredients have been introduced in both the U.S. Senate and the U.S. House of Representatives, and additional proposed legislation is expected to be introduced in 2020. Future legislation approved by Congress and signed by the President, or rulemaking promulgated by the FDA, could either positively or adversely impact the future sale of products by the Company.
| 56 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Additionally, numerous states have passed forms of hemp legislation governing the cultivation of hemp, as well as the further processing and sale of hemp and products with hemp or hemp-derived ingredients. Those states that have not yet enacted laws or issued regulations pertaining to hemp and hemp-derived ingredients may do so in the near future. Until such time as formal federal laws are enacted or regulations are promulgated, we are subject to the laws and regulations in each jurisdiction where we sell products. Changes in the state laws and regulations could again either positively or adversely affect our ability to sell products in those states.
Employees
The Company has 90 full-time employees working out of its headquarters in Henderson, Las Vegas, its Denver Colorado manufacturing facility or individuals’ home-based offices.
Properties
Our executive office and main manufacturing warehouse are located at 1710 Whitney Mesa Drive, Henderson, NV 89014 under a one-year lease. The Company has a second manufacturing facility at 4986 Morrison Rd Denver, CO 80219 under a one-year lease.
Legal Proceedings
There is no material litigation, arbitration or governmental proceeding currently pending against us or any members of our management team in their capacity as such.
Intellectual Property
We not currently have any patents, trademarks or copyrights. We currently rely on the following trade names:
One Hit Wonder, Muffin Man, My Man, Magic Man, Police Man, Rocket Man, Island Man, Army Man, Made Man, One Hit Wonder Cannoli Series, One Hit Wonder Popsicle Series, One Hit Wonder E-Liquid, Wonder-Wire, One Hot Wonder E-Liquid, Turnic, and The Man.
We rely on a combination of trade secret, including federal, state and common law rights in the United States, nondisclosure agreements, and other measures to protect our intellectual property. We require our employees, consultants, and advisors to execute confidentiality agreements and to agree to disclose and assign to us all inventions conceived under their respective employment, consultant, or advisor agreement, using our property, or which relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary. Our business is affected by our ability to protect against misappropriation and infringement of our intellectual property, including our trademarks, service marks, patents, domain names, copyrights and other proprietary rights.
| 57 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
MANAGEMENT
All directors of our company hold office until the next annual meeting of the security holders or until their successors have been elected and qualified. The officers of our company are appointed by our board of directors and hold office until their death, resignation or removal from office. Our directors and executive officers, their ages, positions held, and duration as such, are as follows:
|
Name |
|
Position Held with the Company |
|
Age |
|
Date First Elected or Appointed |
|
Allan Marshall |
|
Chief Executive Officer, Chairman of the Board |
|
54 |
|
May 17, 2019 |
|
Robert Hackett |
|
President |
|
37 |
|
August 5, 2018 |
|
Andrew Norstrud |
|
Chief Financial Officer, Director |
|
47 |
|
April 1, 2020 |
|
Gene Salkind |
|
Director |
|
67 |
|
January 1, 2021 |
|
Thomas C. Williams |
|
Director |
|
61 |
|
January 1, 2021 |
|
Lawrence H Dugan |
|
Director |
|
54 |
|
January 1, 2021 |
Business Experience
The following is a brief account of the education and business experience during at least the past five years of each director, executive officer and key employee of our company, indicating the person’s principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
Allan Marshall, 53, Chief Executive Officer, Director. Mr. Marshall was retired prior to joining the Company working as a serial entrepreneur with a focus on development stage companies in hyper growth industries, with the past several years focusing on the technology and cannabis industries. Mr. Marshall is often the driving force behind the organization for its initial growth and funding strategies. Mr. Marshall began his career in the transportation and logistics industry. Mr. Marshall founded Segmentz, Inc. in November of 2000 and served as the Chief Executive Officer, successfully acquiring five distinct logistic companies, raised more than $25,000,000 of capital, creating the infrastructure and business foundation that is now XPO Logistics, Inc. (XPO NYSE) with revenues in excess of $17 billion. Prior to Segmentz, Mr. Marshall founded U.S. Transportation Services, Inc. (“UST”) in 1995, whose main focus was third party logistics. UST was sold to Professional Transportation Group, Inc. in January 2000 and Professional Transportation Group ceased business in November 2000. Prior to 1995, Mr. Marshall served as Vice President of U.S. Traffic Ltd, a Canadian company, where he founded their United States logistics division and had previously founded a successful driver leasing company in Toronto, Ontario, Canada.
Robert Hackett, 37, President. Mr. Hackett has been actively building consumer lifestyle businesses for 15 years. In 2004, he opened a hookah lounge in Whittier, California. Prior to joining as an executive of the Company, Mr. Hackett worked for Havz LLC, a Company acquired by the Company in 2019. By 2010 he had opened three lounges and had started a distribution business for related products to other lounges and retailers throughout California. In 2011, his firm entered into an exclusive contract to distribute a tobacco-free hookah alternative invented in Germany, called Shiazo. He retained full rights to North America. Over the next few years, as the retail footprint of customers purchasing the Shiazo product increased, Robb’s Company added products to its distribution portfolio which could be sold into the expanding customer base. In 2014, Robb’s Company started formulating and manufacturing its own vape liquid line, “One Hit Wonder”, which over the next two years, became a globally recognized vape eliquid brand. Over the next few years, Robb led the development of several additional brands and more than 100 SKU’s, including the launch of cannabidiol (hemp derived CBD) products. Robb recognized the need for a more efficient sourcing and distribution model, and the potential of building one online. He hired a team of programmers and developers and built a dropship platform that would enable vendors and buyers to seamlessly transact. CBD.io was created as a singular destination for vendors operating in the burgeoning CBD market to source, private-label, wholesale and retail. The platform design is a response to solving the issues of inefficiency and cost that he had experienced in the vape and CBD supply chain over the past several years.
| 58 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Andrew J. Norstrud, 47, Chief Financial Officer, Director. Mr. Norstrud joined Grove, Inc. in July of 2019 as a consultant and became the Chief Financial Officer in April of 2020 and a Director as of January, 2020. Mr. Norstrud is also the Chief Financial Officer and Director of nDivsion Inc. since January of 2019 and working with nDivsion Inc. since March of 2018. Prior to joining Grove, Inc., Mr. Norstrud served as the Chief Financial Officer for Gee Group Inc. from April 1, 2015 until June 15, 2018. Mr. Norstrud joined the Company in March 2013 as CFO and served as CEO and CFO from March 7, 2014 until April 1, 2015. Mr. Norstrud served as a director of GEE Group Inc. from March 7, 2014 until August 16, 2017. Prior to GEE Group Inc., Mr. Norstrud was a consultant with Norco Accounting and Consulting from October 2011 until March 2013. From October 2005 to October 2011, Mr. Norstrud served as the Chief Financial Officer for Jagged Peak. Prior to his role at Jagged Peak, Mr. Norstrud was the Chief Financial Officer of Segmentz, Inc. (XPO Logistics), and played an instrumental role in the company achieving its strategic goals by pursuing and attaining growth initiatives, building a financial team, completing and integrating strategic acquisitions and implementing the structure required of public companies. Previously, Mr. Norstrud worked for Grant Thornton LLP and PricewaterhouseCoopers LLP and has extensive experience with young, rapid growth public companies. Mr. Norstrud earned a BA in Business and Accounting from Western State College and a Master of Accounting with a systems emphasis from the University of Florida. Mr. Norstrud is a Florida licensed Certified Public Accountant.
Gene Salkind, 67, Director. Gene Salkind, M.D. has been a practicing neurosurgeon for greater than 35 years outside of Philadelphia, PA. He graduated from the University of Pennsylvania in 1974 with a B.A., Cum Laude, and received his medical degree from the Lewis Katz School of Medicine in 1979. He returned to the University of Pennsylvania for his neurosurgical residency and in 1985 was selected as the Chief Resident in Neurosurgery at the Hospital of the University of Pennsylvania. Since that time, he has been in a University affiliated practice of general neurological surgery. He is currently the Chief of Neurosurgery at Holy Redeemer Hospital and has also been the Chief of Neurosurgery at Albert Einstein Medical Center and Jeanes Hospital in Philadelphia. He has authored numerous peer reviewed journal articles and has given lectures throughout the country on various neurosurgical topics. He has held professorships at the University of Pennsylvania, the Allegheny Health Education and Research Foundation, and currently at the Lewis Katz School of Medicine.
Dr. Salkind is a prominent investor in the pharmaceutical arena. Past investments include Intuitive Surgical, Pharmacyclics, which grew from less than $1 per share to subsequently being acquired by Abbvie for $250 per share, and Centocor, one of the nation’s largest biotechnology companies, which was acquired by Johnson & Johnson for $4.9 billion in stock. Dr. Salkind currently sits on the boards of Cure Pharmaceuticals, a leader in the biotechnology field through its continual pursuit of redefining traditional drug delivery, and Mobiquity Technologies, Inc., a digital engagement provider. The company owns and operates a national location based mobile advertising network. The company’s suite of technologies allows clients to execute personalized and relevant experiences, driving brand awareness and incremental revenue. He was previously a board member of Derm Tech international, a global leader in non-invasive dermatological molecular diagnostics.
Dr. Salkind in 2019 joined the Strategic Advisory Board of Bio Symetrics, a company that has built data services tools for automated pre-processing, integrated analytics, and predictive modeling to make machine learning accessible to scientists and providers. Their technology serves health and hospital systems, biopharma, drug discovery and precision medicine. Dr. Salkind is and has been an employee and shareholder of Leonard A. Bruno MD/ Gene Salkind MD for the past five years.
| 59 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Thomas Williams, 61, has over 35 years of experience in the insurance industry. He has served in multiple roles in both originations and the administration side of operations. Mr. Williams has a specialization in providing securitization mechanisms of illiquid insurance assets. Thomas was with Smith Barney for his training on the capital markets and insurance industries.
Mr. Williams is currently an Officer and Director in several Ireland based holding companies with a focus in the insurance industry. He is an acting member of the Risk Committee of Wyndham, a large Bermuda based captive. Additionally, he has formed three insurance operations: JTRM, GIH and Arculius. Their lines of business range from Directors and Officers Liability Coverage, Life Extension Risk and Workers Compensation. He has extensive experience in the Offshore and European Union insurance markets in both developing the structure and implementing corporate governance.
Mr. Williams was the intermediary in the sale of Associate Industries of Florida, one of the largest insurance companies in Workers Compensation. He facilitated the sale to Am Trust, a New York publicly traded company in 2009.
Mr. Williams has served on the Board of two public companies:
|
|
· |
GEE Group, an American Stock Exchange Company from 2008 to 2018. At this company, he chaired the nominating committee and was a member of the Corporate Governance Committee and Audit Committee. |
|
|
|
|
|
|
· |
Two Rivers Water and Farming from 2019 to 2020. |
Mr. Williams completed a training program at Northwestern’s Kellogg Business School for Corporate Governance in Public Companies in 2013.
Lawrence H Dugan, 54, Director. Mr. Dugan is a partner with the accounting firm Dorra & Dugan and has been since 1996. Mr. Dugan graduated from the University of Central Florida in 1989. Mr. Dugan is a Florida licensed Certified Public Accountant.
Employment Agreements
On May 17, 2019, the Company entered an employment agreement with Allan Marshall, Chairman and Chief Executive Officer (the “Marshall Employment Agreement”). The Marshall Employment Agreement provides for a three-year term ending on May 17, 2022, unless employment is earlier terminated in accordance with the provisions thereof and after the initial term has a standard 1-year automatic extension clause if there is no notice by the Company of termination. Mr. Marshall received a starting base salary at the rate of $300,000 per year which can be adjusted by the Compensation Committee. Mr. Marshall was granted an option to purchase 2,000,000 shares of Common Stock at a price of $0.85 per share with 1,000,000 shares vesting immediately and 1,000,000 shares vesting ratably over a two-year period. The options are exercisable for 10 years and provide for cashless exercise. Mr. Marshall is entitled to receive an annual bonus based on criteria to be agreed to by Mr. Marshall and the Compensation Committee. The Marshall Employment Agreement contains standard termination, change of control, non-compete and confidentiality provisions.
On July 1, 2020, the Company entered an employment agreement with Nate Weinberg, Chief Sales and Marketing Officer (the “Weinberg Employment Agreement”). The Weinberg Employment Agreement provides for a two-year term ending on July 1, 2022, unless employment is earlier terminated in accordance with the provisions thereof. Mr. Weinberg received a starting base salary at the rate of $120,000 per year which can be adjusted by the Compensation Committee and can achieve bonuses during the term of the agreement. Mr. Weinberg was granted an option to purchase two hundred thousand shares of Common Stock at a price of $0.85 per share vesting ratably over a two-year period. The options are exercisable for 10 years and provide for cashless exercise. The Weinberg Employment Agreement contains standard termination, change of control, non-compete and confidentiality provisions.
| 60 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
On July 1, 2020, the Company entered an employment agreement with Joseph Reid, Director of Operations (the “Reid Employment Agreement”). The Reid Employment Agreement provides for a two-year term ending on July 1, 2022, unless employment is earlier terminated in accordance with the provisions thereof. Mr. Reid received a starting base salary at the rate of $90,000 per year which can be adjusted by the Compensation Committee and achieve bonuses during the term of the agreement. Mr. Reid was granted an option to purchase two hundred thousand shares of Common Stock at a price of $0.85 per share vesting ratably over a two-year period. The options are exercisable for 10 years and provide for cashless exercise. The Reid Employment Agreement contains standard termination, change of control, non-compete and confidentiality provisions.
On February 1, 2021, the Company entered an employment agreement with Andrew Norstrud, Chief Financial Officer (the “Norstrud Employment Agreement”). The Norstrud Employment Agreement provides for a three-year term ending on February 1, 2023, unless employment is earlier terminated in accordance with the provisions thereof and after the initial term has a standard 1-year automatic extension clause if there is no notice by the Company of termination. Mr. Norstrud received a starting base salary at the rate of $250,000 per year which can be adjusted by the Compensation Committee. Mr. Norstrud was granted an option to purchase seven hundred thousand shares of Common Stock at a price of $0.85 per share vesting ratably over a two-year period. The options are exercisable for 10 years and provide for cashless exercise. Mr. Norstrud is entitled to receive an annual bonus based on criteria to be agreed to by Mr. Norstrud and the Chief Executive Officer and the Compensation Committee. The Norstrud Employment Agreement contains standard termination, change of control, non-compete and confidentiality provisions.
Family Relationships
There are no family relationships between any of our directors, executive officers and proposed directors or executive officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years:
|
|
1. |
been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
|
|
|
|
|
|
2. |
had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
|
|
|
|
|
|
3.
|
been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
|
|
|
|
|
|
4.
|
been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
|
|
|
|
|
|
5.
|
been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
|
|
|
|
|
|
6.
|
been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26)), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29)), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
| 61 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Code of Business Conduct and Ethics
The Company has adopted a Code of Business Conduct which is filed as exhibit 14.2.
Limitations on Liabilities and Indemnification of Directors and Officers
For information concerning limitations of liability and indemnification and advancement rights applicable to our directors and officers, see “Description of Capital Stock—Limitations on Liability, Indemnification of Directors and Officers, and Insurance.”
EXECUTIVE AND DIRECTOR COMPENSATION
The particulars of the compensation paid to the following persons:
|
|
(a) |
our principal executive officers; |
SUMMARY COMPENSATION TABLE
|
Name and Principal Position |
|
Year |
|
Salary ($) |
|
|
Bonus ($) |
|
|
Stock Awards ($) |
|
|
Option Awards ($) |
|
|
Non-Equity Incentive Plan Compensation ($) |
|
|
Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) |
|
|
All Other Compensation ($) |
|
|
Total ($) |
| ||||||||
|
Allan Marshall, CEO, and Director |
|
2020 |
|
|
300,000 |
|
|
|
- |
|
|
|
- |
|
|
|
1,325,600 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,625,600 | (1) |
|
|
|
2019 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
Andrew Norstrud, Chief Financial Officer |
|
2020 |
|
|
184,230 |
|
|
|
- |
|
|
|
- |
|
|
|
198,840 |
|
|
|
|
|
|
|
|
|
|
|
|
392,300 | (2) | |||
|
|
|
2019 |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Robert Hackett, President |
|
2020 |
|
|
130,913 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
130,913 |
|
|
|
|
2019 |
|
|
98,364 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
98,364 |
|
| 62 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. Our directors and executive officers may receive share options at the discretion of our board of directors in the future. We do not have any material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that share options may be granted at the discretion of our board of directors. The value of the option awards is based on the intrinsic value at date of grant.
|
|
(1) |
At June 30, 2020 Allan Marshall had an accrual of $72,692 of earned compensation that had not been paid. |
|
|
|
|
|
|
(2) |
For the fiscal year 2020, Andrew Norstrud received compensation through a consulting contract $175,000 and at June 30, 2020 there was an accrual of $7,500 owed to Andrew Norstrud for compensation. |
Outstanding Equity Awards at Fiscal Year-End
Outstanding Equity Awards at Fiscal Year- End Table
The following table summarizes equity awards granted to Named Executive Officers and directors that were outstanding as of September 30, 2020:
|
|
|
Option Awards |
|
Stock Awards |
| |||||||||||||||||||||||||||||
|
Name |
|
Number of Securities Underlying Unexercised Options: # Exercisable |
|
|
Number of Securities Underlying Unexercised Options: # Unexercisable |
|
|
Equity Incentive Plan Awards: Number of Securities Underlying Unearned and Unexercisable Options: |
|
|
Option Exercise Price $ |
|
|
Option Expiration Date |
|
# of Shares or Units of Stock That Have Not Vested # |
|
|
Market Value of Shares or Units of Stock That Have Not Vested $ |
|
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested # |
|
|
Equity Incentive Plan Awards: Market of Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested $ |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Allan Marshall, CEO, and Director |
|
$ | 1,340,006 |
|
|
$ | 150,994 |
|
|
|
|
$ | 0.85 |
|
|
6/1/2029 |
|
|
|
|
|
|
|
| ||||||||||
|
Andrew Norstrud, Chief Financial Officer and Director |
|
|
225,000 |
|
|
|
75,000 |
|
|
|
|
|
$ | 0.85 |
|
|
6/1/2029 |
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Robert Hackett, President |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Gene Salkind, Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Tomas C. Williams, Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Lawrence H Dugan, Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
| 63 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Option Exercises and Stock Vested
In October 2019, Allan Marshall exercised an option to purchase 500,000 shares of Common Stock at a $0.85 per common share. The Company received $400,000 of cash and was relieved of $25,000 in payables to Allan Marshall for the shares of Common Stock.
Compensation of Directors
We do not have any agreements for compensating our directors for their services in their capacity as directors, although such directors are expected in the future to receive stock options to purchase shares of our Common Stock as awarded by our board of directors.
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors or executive officers or any associate or affiliate of our company during the last two fiscal years, is or has been indebted to our company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
| 64 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Except as disclosed herein, no director, executive officer, shareholder holding at least 5% of shares of our Common Stock, or any family member thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction during the year ended June 30, 2020, in which the amount involved in the transaction exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at the year-end for the last three completed fiscal years.
For the year ended June 30, 2020 and June 30, 2019, the Company leased the Las Vegas warehouse from a shareholder for $22,071 per month. This lease ended December 31, 2019 and there were no further liabilities related to this lease. The owner of the warehouse is also related to one of the members of management.
Director Independence
The Board of Directors has determined that Gene Salkind, Lawrence Dugan and Thomas Williams are independent directors under the listing standards.
Promoters and Control Persons
None
| 65 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Immediately prior to this offering, there 12,008,333 shares of our Common Stock outstanding. The following table and accompanying footnotes set forth certain information with respect to the beneficial ownership of our Common Stock, immediately prior to and immediately after the completion of this offering, by:
|
|
· |
each of our directors and named executive officers; |
|
|
|
|
|
|
· |
all of our directors and named executive officers as a group; and |
|
|
|
|
|
|
· |
each person or entity (or group of affiliated persons or entities) known by us to be the beneficial owner of 5% or more of our Common Stock. |
To our knowledge, each stockholder named in the table has sole voting and investment power with respect to all of the shares shown as beneficially owned by such stockholder, except as otherwise set forth in the footnotes to the table. The number of shares shown represents the number of shares the person “beneficially owns,” as determined by the rules of the SEC. The SEC has defined “beneficial” ownership of a security to mean the possession, directly or indirectly, of voting power and/or investment power. A stockholder is also deemed to be, as of any date, the beneficial owner of all securities that such stockholder has the right to acquire within 60 days after that date through (i) the exercise of any option, warrant or right, (ii) the conversion of a security, (iii) the power to revoke a trust, discretionary account or similar arrangement, or (iv) the automatic termination of a trust, discretionary account or similar arrangement.
The percentages reflect beneficial ownership immediately prior to and immediately after the completion of this offering as determined in accordance with Rule 13d-3 under the Exchange Act, and are based on 12,008,333 shares of our Common Stock outstanding as of the date immediately prior to the completion of this offering, and 14,208,333 shares of our Common Stock outstanding as of the date immediately following the completion of this offering. The percentages assume no exercise by the underwriters of their option to purchase up to an additional shares of our Common Stock within 30 days after the date of this prospectus.
Except as noted in the footnotes to the table below, the address for all of the stockholders in the table below is c/o Grove, Inc. at 1710 Whitney Mesa Drive, Henderson, NV 89014.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the ownership, as of February 8, 2021 of our common stock by each of our directors, by all of our executive officers and directors as a group and by each person known to us who is the beneficial owner of more than 5% of any class of our securities. As of February 8, 2021, there were 12,008,333 shares of our common stock issued and outstanding. All persons named have sole or shared voting and investment control with respect to the shares, except as otherwise noted. The number of shares described below includes shares which the beneficial owner described has the right to acquire within 60 days of the date of this prospectus. Unless otherwise indicated, the address for each beneficial owner is c/o Grove, Inc., 1710 Whitney Mesa Drive, Henderson, NV 89014.
|
Name and Address of Beneficial Owner |
|
Amount and Nature of Beneficial Ownership |
|
|
Percentage of Class(1) |
| ||
|
Allan Marshall |
|
|
3,598,766 |
(2) |
|
|
27.51 |
% |
|
Gene Salkind |
|
|
2,357,571 |
(3) |
|
|
19.63 |
% |
|
Robert Hackett |
|
|
1,444,444 |
(4) |
|
|
12.03 |
% |
|
Andrew Norstrud |
|
|
492,592 |
(5) |
|
|
4.04 |
% |
|
Lawrence Dugan |
|
|
4,630 |
(6) |
|
|
* |
% |
|
Thomas Williams |
|
|
4,630 |
(7) |
|
|
* |
% |
|
Directors and Executive Officers as a Group |
|
|
7,902,632 |
|
|
|
65,81 |
% |
|
|
|
|
|
|
|
|
|
|
|
5% or more Stockholders |
|
|
|
|
|
|
|
|
|
Jeffrey Bishop |
|
|
1,016,340 |
|
|
8.46 |
% | |
|
Nikolaos Voudouris |
|
|
777,778 |
|
|
|
6.48 |
% |
__________
|
* |
Represents less than 1% of the number of shares of our Common Stock outstanding |
|
(1) |
Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the number of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding on February 8, 2021. As of February 8, 2021, there were 21,615,000 shares of our company’s common stock issued and outstanding. |
|
(2) |
Represents (i) 2,527,778 shares of common stock, (ii) 793,210 shares issuable upon the exercise of stock options that are exercisable within 60 days, (iii) 277,778 shares issuable upon the conversion of preferred stock. Does not include 40,123 shares issuable upon vesting and exercise of remaining stock option. |
|
(3) |
Represents (i) 2,352,941 shares of common stock and (ii) 4,630 shares issuable upon the exercise of stock option that are exercisable within 60 days. Does not include 23,148 shares issuable upon vesting and exercise of remaining stock option. |
|
(4) |
Represents 1,444,444 shares of common stock. |
|
(5) |
Represents (i) 305,556 shares of common stock and (ii) 187,036 shares issuable upon the exercise of stock options that are exercisable within 60 days. Does not include 368,519 shares issuable upon vesting and exercise of remaining stock options. |
|
(6) |
Represents 4,360 shares issuable upon the exercise of stock option that are exercisable within 60 days. Does not include 23,148 shares issuable upon vesting and exercise of remaining stock option. |
|
(7) |
Represents 4,360 shares issuable upon the exercise of stock option that are exercisable within 60 days. Does not include 23,148 shares issuable upon vesting and exercise of remaining stock option. |
| 66 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
DESCRIPTION OF CAPITAL STOCK
General
The following is a description of the material terms of, and is qualified in its entirety by, our certificate of incorporation and bylaws, each of which will be in effect upon the consummation of this offering, the forms of which are filed as exhibits to the registration statement of which this prospectus forms a part. Under “Description of Capital Stock,” “we,” “us,” “our,” the “Company” and “our company” refer to Grove Inc. and not to any of its subsidiaries.
Common Stock
We are authorized to issue up to 100,000,000 shares of Common Stock at a par value of $0.001 per share. As of February 2, 2021, there were 12,008,333 shares of Common Stock outstanding. The holders of Common Stock will have the right to vote on all matters on which stockholders have the right to vote, and holders of Common Stock shall be entitled to one (1) vote per share.
Holders of Common Stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding Preferred Stock.
In the event of our liquidation or dissolution, the holders of Common Stock are entitled to receive proportionately all assets available for distribution to shareholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of Common Stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of Preferred Stock that we may designate and issue in the future.
Our bylaws authorize the Board of to provide for the issuance of shares of Preferred Stock in series and, by filing a certificate pursuant to the Nevada Revised Statutes (“N.R.S.”), to establish from time to time one or more classes of Preferred Stock or one or more series of Preferred Stock, by fixing and determining the number of shares to be included in each such class or series, and to fix the designation, powers, preferences and rights of the shares of each such series and the qualifications, limitations and restrictions thereof.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of Preferred Stock at a par value of $0.001 per share. As of February 2, 2021, there were 500,000 shares of Series A Preferred Stock outstanding. The holders of Series A Preferred Stock will have the right to vote on all matters on which stockholders have the right to vote, and holders of Series A Preferred Stock shall be entitled to ten (10) votes per share and shall vote together as a separate class on stock on all matters which impact the rights, value, or ranking of the Common Stock or Series A Preferred Stock.
Each share of Series A Preferred Stock is convertible into one (1) share of Common Stock, at any time at the request of the holder of Series A Preferred Stock
In the event of our liquidation, consolidation, merger or dissolution, the holders of Series A Preferred Stock are entitled to receive an amount on such date equal to the Stated Value of Series A Preferred Stock, which is $0.05 per share.
Anti-Takeover Provisions
We are governed by the provisions of Nevada Revised Statutes 78.378 to 78.3793 because we are incorporated in Nevada. Nevada’s “combinations with interested stockholders” statutes (NRS 78.411 through 78.444, inclusive) prohibit specified types of business “combinations” between certain Nevada corporations and any person deemed to be an “interested stockholder” for two years after such person first becomes an “interested stockholder” unless the corporation’s board of directors approves the combination (or the transaction by which such person becomes an “interested stockholder”) in advance, or unless the combination is approved by the board of directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an “interested stockholder” is any person who is (1) the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the outstanding voting shares of the corporation, or (2) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the then-outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to cover most significant transactions between a corporation and an “interested stockholder.”
| 67 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Nevada’s “acquisition of controlling interest” statutes (NRS 78.378 through 78.3793, inclusive) contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These laws would apply to us if we were to have 200 or more stockholders of record (at least 100 of whom have addresses in Nevada appearing on our stock ledger) and do business in the State of Nevada directly or through an affiliated corporation, unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the NRS, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described above apply.
In addition, NRS 78.139 also provides that directors may resist a change or potential change in control if the directors, by majority vote of a quorum, determine that the change is opposed to, or not in, the best interests of the corporation.
Any provision in our articles of incorporation or bylaws or Nevada law that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
Limitations on Liability, Indemnification of Directors and Officers, and Insurance
In accordance with the NRS and pursuant to the bylaws of the Company, subject to certain conditions, the Company shall, to the maximum extent permitted by law, indemnify a director or officer, a former director or officer, or another individual who acts or acted at the Company’s request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including any amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the Company or other entity. We shall advance monies to a director, officer or other individual for costs, charges and expenses actually and reasonably incurred in connection with such a proceeding; provided that such individual shall repay the moneys if the individual does not fulfill the conditions described below or is not successful on the merits in their defense of the action or proceeding.
Indemnification is prohibited unless the individual:
|
|
· |
Acted honestly and in good faith, on an informed basis and with a view to our best interests; |
|
|
|
|
|
|
· |
In the case of a criminal or administration action or proceeding enforced by a monetary penalty, had reasonable grounds to believe the conduct was lawful; and |
|
|
|
|
|
|
· |
Was not judged by a court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done. |
Authorized but Unissued Shares
The authorized but unissued shares of our Common Stock are available for future issuance without shareholder approval, subject to any limitations imposed by the listing standards of The Nasdaq Capital Market. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar
The transfer agent and registrar for shares of our Common Stock will be [___________].
Listing
We expect to apply to list our Common Stock on The Nasdaq Capital Market under the symbol “[______].”
| 68 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SHARES ELIGIBLE FOR FUTURE SALE
General
Upon the completion of this offering, as a result of the issuance of shares of our Common Stock in this offering, we expect that there will be shares of our Common Stock issued and outstanding ( shares if the underwriters exercise in full their option to purchase additional shares of our Common Stock in this offering).
Of the total number of shares of our Common Stock to be issued and outstanding upon completion of this offering:
|
|
· |
shares are being offered and sold in this offering ( shares if the underwriters exercise in full their option to purchase additional shares in this offering). These shares will be freely transferable without restriction or further registration under the Securities Act, except that any shares held or acquired by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, will be subject to the volume limitations and other restrictions of Rule 144 described below; and |
|
|
|
|
|
|
· |
the remaining shares have not been registered and may be “restricted” securities within the meaning of Rule 144 under the Securities Act. These shares may not be sold unless they are registered under the Securities Act, the restrictions under Rule 144 have lapsed, or another exemption from registration is available. In addition, these shares are subject to lock-up agreements for 60 days after the effective date of the registration statement of which this prospectus forms a part (or for 180 days after the effective date of the registration statement of which this prospectus forms a part in the case of any such shares held by any of our directors or officers). |
Prior to this offering, there has been no public market for shares of our Common Stock. Although we intend to apply to list the shares of our Common Stock on the [___________] under the symbol “[____],” an active trading market for our Common Stock may never develop or, if one develops, it may not be sustained following this offering. No assurance can be given as to the likelihood that an active trading market for our Common Stock will develop, the liquidity of any such market, the ability of our stockholders to sell their shares, or the prices that our stockholders may obtain for any of their shares. No prediction can be made as to the effect, if any, that future sales of shares of our Common Stock, or the availability of shares of our Common Stock for future sale, will have on prevailing market prices from time to time. Sales of substantial amounts of our Common Stock, or the perception that such sales could occur, may adversely affect prevailing market prices of our Common Stock. See “Risk Factors—Risks Related to this Offering and our Common Stock.”
Rule 144
After the completion of this offering, shares of our outstanding Common Stock will be “restricted” securities under the meaning of Rule 144 under the Securities Act, and may not be sold in the absence of registration under the Securities Act unless an exemption from registration is available, including the exemption provided by Rule 144.
In general, under Rule 144 as currently in effect, beginning 90 days after the completion of this offering, a person who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned shares considered to be restricted securities under Rule 144 for at least six months (including the holding period of any prior owner other than an affiliate), would be entitled to sell those shares, subject only to the availability of current public information about us. A non-affiliated person who has beneficially owned shares considered to be restricted securities under Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
| 69 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
An affiliate of ours who has beneficially owned shares of our Common Stock for at least six months would be entitled to sell, within any three-month period, a number of shares that does not exceed the greater of:
|
|
· |
1% of the number of shares of our Common Stock then outstanding, which will equal approximately shares immediately after this offering; or |
|
|
|
|
|
|
· |
the average weekly trading volume of our Common Stock on Nasdaq Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale; |
provided, in each case, that we are subject to the Exchange Act periodic reporting requirements for at least 90 days before such sale and have filed all required reports during that time period. Such sales by affiliates are also subject to other provisions and requirements of Rule 144 relating to manner of sale, notice, and the availability of current public information about us. To the extent that an affiliate sells shares of our Common Stock, other than pursuant to Rule 144 or a registration statement, the purchaser’s holding period for the purpose of effecting a sale under Rule 144 commences on the date of transfer from such affiliate.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our Common Stock pursuant to a written compensatory plan or contract and who is not deemed to have been our affiliate during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits our affiliates to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701 and are subject to the lock-up agreements described above.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL MATTERS RELATING TO SHARE TRANSFER RESTRICTIONS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN LEGAL ADVISOR REGARDING THE PARTICULAR SECURITIES LAWS AND TRANSFER RESTRICTION CONSEQUENCES OF PURCHASING, HOLDING, AND DISPOSING OF OUR COMMON SHARES OR THE COMMON SHARES, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
Incentive Compensation Plan
The Company established a 2019 Equity Incentive Plan (the “2019 Plan”). The plan grants incentives to select persons who can make, are making and continue to make substantial contributions to the growth and success of the Company, to attract and retain the employment and services of such persons and to encourage and reward such contributions by providing these individuals with an opportunity to acquire or increase stock ownership in the Company through either the grant of options or restructured stock. The 2019 Plan is administered by the Compensation Committee or such other committee as is appointed by the Board of Directors pursuant to the 2019 Plan (the “Committee”). The Committee has full authority to administer and interpret the provisions of the 2019 Plan including, but not limited to, the authority to make all determinations with regard to the terms and conditions of an award made under the 2019 Plan. The maximum number of shares that may be granted under the 2019 Plan is 5,000,000. This number is subject to adjustment to reflect changes in the capital structure or organization of the Company. All stock options outstanding as of December 31, 2020 were non-qualified stock options, had exercise prices equal to the market price on the date of grant and had expiration dates 10 years from the date of grant.
Lock-Up Periods
For a description of certain lock-up periods, see “Underwriting.”
| 70 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a summary of certain material U.S. federal income tax considerations, with respect to the ownership and disposition of our Common Stock, generally applicable to non-U.S. holders (as defined below) who hold our Common Stock as a capital asset (generally, property held for investment) within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (which we refer to as the “Code”). This summary applies only to non-U.S. holders who purchase our Common Stock in this offering and hold our Common Stock as a capital asset (generally, property held for investment purposes). This summary does not address all aspects of U.S. federal income taxation that may be relevant to particular non-U.S. holders in light of their individual circumstances or the U.S. federal income tax consequences applicable to non-U.S. holders that are subject to special rules, such as controlled foreign corporations, passive foreign investment companies, underwriters, corporations that accumulate earnings to avoid U.S. federal income tax, banks or other financial institutions, insurance companies, tax-exempt organizations (including private foundations), tax qualified retirement plans, individual retirement accounts or other tax deferred accounts, U.S. expatriates and certain former citizens or residents of the United States, brokers, dealers and traders in securities, commodities or currencies, non-U.S. holders that hold shares of our Common Stock as real estate investment companies, regulated investment companies, grantor trusts, persons that received shares of our Common Stock as compensation for performance of services, persons that have a functional currency other than the U.S. dollar, and persons that own, or have owned, actually or constructively, more than 5% of our Common Stock, or non-U.S. holders that hold shares of our Common Stock as part of a “straddle,” “hedge,” “conversion transaction”, other risk reduction strategy, or as part of a conversion transaction or other integrated investment.
This summary is based on provisions of the Code, U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as in effect on the date hereof, and all of which are subject to change or differing interpretation, possibly on a retroactive basis. The summary does not describe any U.S. state, local or non-U.S. income or other tax consequences (including estate, gift and Medicare contribution tax consequences) of owning and disposing of our Common Stock. No ruling from the Internal Revenue Service (which we refer to as the “IRS”) has been or will be sought with respect to the matters discussed below and the conclusions reached in the following summary, and there can be no assurance that the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of shares of our Common Stock, or that any such contrary position would not be sustained by a court.
For purposes of this summary, the term “non-U.S. holder” means a beneficial owner of our Common Stock that is, for U.S. federal income tax purposes, neither a partnership (or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) nor any of the following:
|
|
· |
a citizen or individual resident of the United States; |
|
|
|
|
|
|
· |
a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
|
|
|
|
|
|
· |
an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
|
|
|
|
|
|
· |
a trust if (a) a United States court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (b) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
If a partnership (including any entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds our Common Stock, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. Partnerships holding our Common Stock, and partners in such partnerships, should consult their tax advisers as to the U.S. federal income tax consequences applicable to them in their particular circumstances.
| 71 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
EACH NON-U.S. HOLDER IS URGED TO CONSULT ITS TAX ADVISER REGARDING THE U.S. FEDERAL, STATE, LOCAL, AND NON-U.S. INCOME AND OTHER TAX CONSEQUENCES OF OWNING AND DISPOSING OF OUR COMMON STOCK.
Distributions on Common Stock
Distributions on our Common Stock generally will be treated as dividends to the extent paid from our current or accumulated earnings and profits as determined for U.S. federal income tax purposes. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated first as a return of capital and will first be applied to reduce a non-U.S. holder’s adjusted tax basis in our Common Stock (but not below zero), and thereafter will be treated as capital gain from the sale or exchange of such Common Stock, subject to the tax treatment described below in “—Sale, Exchange or Other Taxable Disposition of Common Stock”. Generally, the gross amount of dividends paid to a non-U.S. holder with respect to our Common Stock will be subject to withholding of U.S. federal income tax at a rate of 30%, or at a lower rate if an applicable income tax treaty so provides and we (or our agent) have received proper certification as to the application of that treaty.
Dividends that are effectively connected with a non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable tax treaty, are attributable to a U.S. permanent establishment of the non-U.S. holder) are generally subject to U.S. federal income tax on a net income basis and are exempt from the 30% withholding tax described above (assuming compliance with certain certification requirements). Any such effectively connected dividends received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a rate of 30% (or lower applicable treaty rate).
To claim the benefit of an applicable tax treaty or an exemption from withholding because the income is effectively connected with the conduct of a trade or business in the United States, a non-U.S. holder generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (if the holder is claiming the benefits of an income tax treaty) or IRS Form W-8ECI (for income effectively connected with a trade or business in the United States) or other suitable form. If you are a non-U.S. holder, you may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisers regarding their entitlement to benefits under an applicable income tax treaty and the specific manner of claiming the benefits of the treaty.
Sale, Exchange or Other Taxable Disposition of Common Stock
A non-U.S. holder generally will not be subject to U.S. federal income or withholding tax with respect to gain on the sale, exchange or other taxable disposition of our Common Stock unless (i) the gain is effectively connected with such non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable tax treaty, is attributable to a U.S. permanent establishment of such non-U.S. holder), (ii) in the case of a non-U.S. holder that is a non-resident alien individual, such non-U.S. holder is present in the United States for 183 or more days in the taxable year of disposition and certain other requirements are met, or (iii) we are or have been a “United States real property holding corporation” for U.S. federal income tax purposes at any time within the shorter of the five-year period ending on the date of such sale, exchange, or other taxable disposition or the period that such non-U.S. holder held our Common Stock and either (a) our Common Stock was not treated as regularly traded on an established securities market at any time during the calendar year in which the sale, exchange or other taxable disposition occurs, or (b) such non-U.S. holder owns or owned (actually or constructively) more than five percent of our Common Stock at any time during the shorter of the two periods mentioned above. We believe that we are not currently a U.S. real property holding corporation; however, there can be no assurance that we will not become a U.S. real property holding corporation in the future. If we become a U.S. real property holding corporation and our Common Stock is not regularly traded on an established securities market, a non-U.S. holder generally will be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our Common Stock on a net income basis in the same manner as if such holder were a resident of the United States.
| 72 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Unless an applicable tax treaty provides otherwise, if gain or loss is effectively connected with a non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable tax treaty, is attributable to a U.S. permanent establishment of such non-U.S. holder), the non-U.S. holder will be subject to U.S. federal income tax on the disposition of our Common Stock on a net income basis in the same manner in which citizens or residents of the United States would be subject to U.S. federal income tax. In the case of a non-U.S. holder that is a foreign corporation, such gain may also be subject to an additional branch profits tax at a rate of 30% (or a lower applicable treaty rate). If a non-U.S. holder is an individual that is present in the United States for 183 or more days in the taxable year of disposition and certain other requirements are met, the non-U.S. holder generally will be subject to a flat income tax at a rate of 30% (or lower applicable treaty rate) on any capital gain recognized on the disposition of our Common Stock, which may be offset by certain U.S. source capital losses.
Information Reporting and Backup Withholding
You generally will be required to comply with certain certification procedures to establish that you are not a U.S. person (for instance, furnishing to us a properly executed applicable IRS Form W-8, such as an IRS Form W-8BEN, W-8BEN-E or IRS Form W-8ECI) in order to avoid backup withholding with respect to dividends or the proceeds of a sale, exchange or other taxable disposition of common stock. Notwithstanding the foregoing, information reporting and backup withholding may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person. In addition, we are required to annually report to the IRS and you the amount of any dividends paid to you, regardless of whether we actually withheld any tax. Copies of the information returns reporting such dividends and the amount withheld may also be made available to the tax authorities in the country in which you reside under the provisions of an applicable income tax treaty. Any amounts withheld under the backup withholding rules generally are not an additional tax and may be allowed as a refund or credit against your U.S. federal income tax liability, provided that certain required information is provided on a timely basis to the IRS.
Foreign Account Tax Compliance Act
Withholding taxes may be imposed under the Foreign Account Tax Compliance Act (which we refer to as “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, subject to certain exceptions, withholding at a rate of 30% generally will be required (a) on dividends paid with respect to shares of our Common Stock, and (b) subject to the following sentence, in respect of gross proceeds from the sale or other disposition of, shares of our Common Stock, in each case, paid to (i) a non-financial non-U.S. entity, unless such entity either certifies to us or our paying agent that such entity does not have any “substantial United States owners” or provides certain information regarding the entity’s “substantial United States owners”, which we will in turn provide to the U.S. Department of the Treasury, or (ii) certain non-U.S. financial institutions (including investment funds), unless such institution enters into, and complies with, an agreement with the IRS to report, on an annual basis, information with respect to interests in, or accounts maintained by, the institution that are owned by certain U.S. persons or by certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or if required under an intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority, which will exchange such information with the U.S. authorities. The IRS and the U.S. Treasury Department issued proposed U.S. Treasury Regulations (which we refer to as the “Proposed Regulations”) that would remove gross proceeds described in (b) above from the withholding obligation. Taxpayers may rely on the Proposed Regulations until final regulations are issued. Under certain circumstances, a Non-U.S. Holder of shares of our Common Stock might be eligible for refunds or credits of the withholding tax. Prospective investors are encouraged to consult with their tax advisors regarding the potential application of withholding under FATCA to their investment in our Common Stock. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Prospective investors should consult with their tax advisers regarding the possible implications of these rules on their investment in our Common Stock, including without limitation, the interaction of FATCA withholding with the other withholding rules discussed above.
| 73 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Kingswood Capital Markets, division of Benchmark Investments, Inc., is acting as the representative of the underwriters of the Offering (the “Representative”). We intend to enter into an underwriting agreement dated _____, 2021 with the Representative. We plan to list our common stock for trading on the Nasdaq Capital Market under the symbol “ ” in connection with this Offering, although there is no assurance that Nasdaq will approve our initial listing application for our common stock. If we fail to obtain such Nasdaq approval or to list our common stock on the New York Stock Exchange, we will not be able to consummate the Offering and will terminate this Offering. Subject to the terms and conditions of the underwriting agreement, we intend to sell to each underwriter named below, and each underwriter named below intends to severally purchase, at the public offering price, less the underwriting discounts set forth on the cover page of this prospectus, the number of units listed next to its name in the following table:
|
Underwriter |
Number of Shares | ||
|
Kingswood Capital Markets, division of Benchmark Investments, Inc. |
|||
|
Total |
|||
A form of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the Representative that they propose to offer the shares directly to the public at the public offering price set forth on the cover page of this prospectus. Any shares sold by the Representative to securities dealers will be sold at the public offering price, less a selling concession not in excess of $ _________ per share. The underwriting agreement will provide that subject to the satisfaction or waiver by the Representative of the conditions contained in the underwriting agreement, the Representative is obligated to purchase and pay for all of the shares offered by this prospectus.
We have granted an option to the Representative exercisable for forty-five (45) days after the date of this prospectus, to purchase up to _______________ additional shares at the public offering price, less the underwriting discounts and commissions.
No action has been taken by us or the Representative that would permit a public offering of the shares in any jurisdiction outside the United States where action for that purpose is required. None of our shares of common stock included in this Offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any such securities offered hereby be distributed or published in any jurisdiction except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this Offering of shares and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the shares in any jurisdiction where that would not be permitted or legal.
The Representative is expected to make offers on sales both in and outside of the United States to its respective selling agents. The offers and sales in the United States will be conducted by broker-dealers registered with the SEC and the Financial Industry Regulatory Authority (FINRA).
The Representative has advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
Underwriting Discount and Expenses
In connection with the Offering, the underwriters shall be granted an underwriting discount of (i) eight percent (8%) for investors introduced by the underwriters and (ii) five percent (5%) for investors introduced by the Company; provided, that if the total gross proceeds from such investors introduced by the Company is less than $3,000,000, then the discount shall instead be eight percent (8%) (the “Underwriting Discount”).
| 74 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Assuming a discount of 8%, the following table provides information regarding the amount of the discount to be paid to the underwriters by us, before expenses:
|
|
|
Per |
|
|
Total options |
|
|
Total exercise of over- options |
| |||
|
Public Offering Price |
|
$ |
|
|
$ |
|
|
$ |
| |||
|
Underwriting discount and commissions (1) |
|
$ |
|
|
$ |
|
|
$ |
| |||
|
Proceeds, before expenses, to us |
|
$ |
|
|
$ |
|
|
$ |
| |||
Assuming a discount of 5%, the following table provides information regarding the amount of the discount to be paid to the underwriters by us, before expenses:
|
|
|
Per |
|
|
Total options |
|
|
Total exercise of over- options |
| |||
|
Public Offering Price |
|
$ |
|
|
$ |
|
|
$ |
| |||
|
Underwriting discount and commissions (1) |
|
$ |
|
|
$ |
|
|
$ |
| |||
|
Proceeds, before expenses, to us |
|
$ |
|
|
$ |
|
|
$ |
| |||
The Company will be also responsible for and will pay all expenses relating to the Offering, including, without limitation, (a) all filing fees and expenses relating to the registration of the securities with the Commission; (b) all fees and expenses relating to the listing of the Company’s common stock on a national exchange, if applicable; (c) all fees, expenses and disbursements relating to the registration or qualification of the securities under the “blue sky” securities laws of such states and other jurisdictions as Kingswood may reasonably designate (including, without limitation, all filing and registration fees, and the reasonable fees and disbursements of the Company’s “blue sky” counsel, which will be Kingswood’s counsel) unless such filings are not required in connection with the Company’s proposed listing on a national exchange, if applicable; (d) all fees, expenses and disbursements relating to the registration, qualification or exemption of the securities under the securities laws of such foreign jurisdictions as Kingswood may reasonably designate; (e) the costs of all mailing and printing of the Offering documents; (f) transfer and/or stamp taxes, if any, payable upon the transfer of securities from the Company to Kingswood; and (g) the fees and expenses of the Company’s accountants; and (h) a maximum of $150,000 for fees and expenses including “road show,” diligence, and reasonable legal fees and disbursements for Kingswood’s counsel. The Company shall be responsible for Kingswood’s external counsel legal costs irrespective of whether or not the Offering is consummated, subject to a maximum of $50,000 in the event that it is not consummated. Additionally, one percent (1%) of the gross proceeds of the Offering shall be provided to Kingswood for non-accountable expenses. Additionally, the Company will provide an expense advance (the “Advance”) to Kingswood of $25,000. The Advance shall be applied towards out-of-pocket accountable expense set forth herein and any portion of the Advance shall be returned to the Company to the extent not actually incurred. Kingswood may deduct from the net proceeds of the Offering payable to the Company on the closing date, or the closing date of the Over-Allotment Option, if any, the expenses set forth herein to be paid by the Company to Kingswood.
We estimate the total expenses payable by us for this Offering to be approximately $ , which amount includes (i) the underwriting discount of $_______ (8%), (ii) reimbursement of the accountable expenses of the representative equal to $150,000 including the legal fees of the representative being paid by us and (iii) other estimated Company expenses of approximately $_________, which includes legal accounting printing costs and various fees associated with the registration of our securities.
| 75 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Underwriters’ Warrants
In addition, we intend to issue warrants to the underwriters to purchase a number of shares of common stock equal to 8% of the total number of shares sold in this Offering at an exercise price equal to 110% of the per share offering price of the shares sold in this Offering (the “Underwriters’ Warrants”). These warrants may be purchased in cash or via cashless exercise, will be exercisable for five years from the effective date of this registration statement of which this prospectus forms a part and will terminate on the fifth anniversary of the effective date of the registration statement on Form S-1 of which this prospectus forms a part. The Underwriters’ Warrants and the shares of common stock issuable upon the exercise of such warrants will be deemed compensation by FINRA, and therefore will be subject to FINRA Rule 5110(g)(1). In accordance with FINRA Rule 5110(g)(1), neither the Underwriters’ Warrants nor any of our shares of common stock issued upon exercise of such warrants may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following the commencement of sales of the securities registered on the registration statement of which this prospectus is a part, subject to certain exceptions.
The Underwriters’ Warrants provide for registration rights (including a one-time demand registration right and unlimited piggyback rights) and customary anti- dilution provisions (for stock dividends and splits and recapitalizations) and anti-dilution protection (adjustment in the number and price of such warrants and the shares underlying such warrants) resulting from corporate events (which would include dividends, reorganizations, mergers, etc.) and future issuance of common stock or common stock equivalents at prices (or with exercise and/or conversion prices) below the Offering price as permitted under FINRA Rule 5110(f)(2)(G). Until the expiration of the Underwriters’ Warrants, the Company will have the right to redeem the Underwriters’ Warrants at any time for 200% of their exercise price.
Determination of Offering Price
The public offering price of the shares offered by this prospectus will be determined by negotiation between us and the Representative. Among the factors considered in determining the public offering price of the shares were:
|
|
· |
our history, capital structure and our business prospects; |
|
|
· |
the industry in which we operate; |
|
|
· |
our past and present operating results; |
|
|
· |
the previous experience of our executive officers; and |
|
|
· |
the general condition of the securities markets at the time of this Offering. |
The offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the shares sold in this Offering. The value of such securities are subject to change as a result of market conditions and other factors.
Subsequent Equity Sales
Pursuant to the underwriting agreement, from the date of such agreement until the first anniversary of the closing date of the Offering, the Representative shall have an irrevocable right of first refusal to act as sole investment banker, sole book-runner, and/or sole placement agent, at the Representative’s sole discretion, for each and every future public and private equity and debt offering, including all equity linked financings (each, a “Subject Transaction”), during such twelve (12) period, of the Company, or any successor to or any current or future subsidiary of the Company, on terms and conditions customary to the Representative for such Subject Transactions. The Representative shall have the sole right to determine whether or not any other broker dealer shall have the right to participate in the Subject Transactions and the economic terms of such participation. For the avoidance of any doubt, the Company shall not retain, engage or solicit any additional investment banker, book-runner, financial advisor, underwriter and/or placement agent in a Subject Transaction without the express written consent of the Representative.
| 76 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Tail Period
In the event that the Offering is not consummated by the Representative as contemplated herein, the Representative shall be entitled to a cash fee equal to eight percent (8%) of the gross proceeds received by the Company from the sale of the securities to any investor actually introduced by the Representative to the Company during the three month period from date of that certain Engagement Agreement, dated September 28, 2020 (the “Engagement Agreement”), between the Company and the Representative (the “Tail Financing”), and such Tail Financing is consummated at any time during the Engagement Period or within the twelve (12) month period following the expiration of the Engagement Period, provided that such financing is by a party actually introduced to the Company in an offering in which the Company has direct knowledge of such party’s participation.
In addition, unless (x) the Company terminates this underwriting agreement for “Cause” (as defined therein), or (y) the Representative fails to provide the underwriting services provided in the underwriting agreement, upon termination of such agreement, if the Company subsequently completes a public or private financing with any investors introduced to the Company by the Representative during the twelve (12) month period following such termination, the Representative shall be entitled to receive the same compensation to be paid to the Representative in connection with this Offering.
Lock-up Agreements
Pursuant to certain “lock-up” agreements, we, our executive officers, directors and securityholders holding more than 5% of the Company’s common stock intend to agree, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, without the prior written consent of the Representative, for a period of 180 days from the date of effectiveness of the offering. In addition, during such period, except for the registration statement of which this prospectus forms a part, such parties have agreed not to file, circulate or participate in the filing or circulation of any registration statement prospectus or other disclosure document with respect to the offer or sale of such securities, or exercise any rights to require registration with the SEC of such securities or offering thereof.
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in stabilizing transactions for the purpose of pegging, fixing or maintaining the price of our common stock. Stabilizing transactions permit bids to purchase the underlying common stock so long as the stabilizing bids do not exceed a specific maximum. These stabilizing transactions may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriters make any representation or prediction as to the effect that stabilizing transactions may have on the price of our common stock. These transactions may be effected on Nasdaq, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time. In connection with this Offering, the underwriters also may engage in passive market making transactions in our common stock in accordance with Regulation M. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriters make any representations or predictions as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriters make any representations that the underwriters will engage in these transactions or that any transactions, once commenced will not be discontinued without notice.
| 77 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the Offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters and selling group members that may make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other financial services for us and our affiliates in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. Pursuant to the Engagement Agreement, dated September 28, 2020, between the Company and the Representative, the Representative agreed to provide general financial advisory services to the Company such as introducing the Company to investors and assisting the Company in financings or other transactions (the “Advisory Services”). Pursuant to the Engagement Agreement, as consideration for the Advisory Services in connection with a private placement of equity securities, the Company shall pay a cash fee of ten percent (10%) of the amount of capital raised, invested or committed and issue to the Representative or its designees at the closing, warrants (the “Placement Agent’s Warrants”) to purchase that number of shares of common stock of the Company equal to ten percent (10%) of the aggregate number of shares of common stock or other equity securities sold in the offering, exercisable at any time and from time to time, in whole or in part, during the four and a half- year period commencing six (6) months from the closing date of the offering, at a price per share equal to 110.0% of the offering price per share of common stock (the “Advisory Fees”). The Placement Agent’s Warrants will provide for registration rights (including a one-time demand registration right and unlimited piggyback rights) and customary anti-dilution provisions (for stock dividends and splits and recapitalizations) and anti-dilution protection (adjustment in the number and price of such warrants and the shares underlying such warrants) resulting from corporate events (which would include dividends, reorganizations, mergers, etc.) and future issuance of common stock or common stock equivalents at prices (or with exercise and/or conversion prices) below the offering price as permitted under FINRA Rule 5110(f)(2)(G). For debt placements, the Company shall pay the Representative a cash fee of eight percent (8%) of the amount of capital raised, invested or committed.
If within twelve (12) months from the effective date of the termination or expiration of the Engagement Agreement either the Company or any party to whom the Company was actually introduced, directly or indirectly, by the Representative, or who was contacted by the Representative on behalf of the Company in connection with its Advisory Services for the Company, proposes a financing (“Financing”) or any a transaction with the Company, including, without limitation, a merger, acquisition or sale of stock or assets (in which the Company may be the acquiring or the acquired entity), joint venture, strategic alliance or other similar transaction (any such transaction, a “Transaction”), then, if any such Financing or Transaction is consummated, the Company shall pay to the Representative the Advisory Fees in cash, or by delivery of the Placement Agent’s Warrant, at the closing or closings of the Financing or Transaction to which it relates. A Financing or Transaction shall be deemed consummated before such date if any agreement in principle which includes material terms of such Financing or Transaction is reached prior to such date even if the closing occurs later.
Indemnification
We intend to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities Act or to contribute to payments that the underwriter may be required to make for these liabilities.
| 78 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The validity of the shares of Common Stock offered hereby will be passed upon for us by Greenberg Traurig, LLP, Sacramento, California. Carmel, Milazzo & Feil, LLP is acting as counsel to the underwriters in connection with the offering.
The financial statements of Grove, Inc. as of and for the year ended June 30, 2020 included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of B F Borgers CPA PC, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
The financial statements of Grove, Inc. as of and for the years ended June 30, 2019 included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of RBSM LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
The financial statements of Infusionz, Inc. as of and for the years ended June 30, 2020 and 2019 included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of B F Borgers CPA PC, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933, as amended, with respect to the shares of our Common Stock being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules which are part of the registration statement. Some items included in the registration statement are omitted from the prospectus in accordance with the rules and regulations of the SEC. For further information about us and our Common Stock offered by this prospectus, we refer you to the registration statement, including all amendments, supplements, exhibits, and schedules thereto. Statements contained in this prospectus regarding the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see a copy of such contract or document that has been filed. Each statement in this prospectus relating to a contract or document that is filed as an exhibit to the registration statement is qualified in all respects by reference to the full text of such contract or document filed as an exhibit to the registration statement.
The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. You can review the registration statement, as well as our future SEC filings, by accessing the SEC’s website at www.sec.gov.
Upon the completion of this offering, we will be subject to the information and periodic reporting requirements of the Exchange Act, applicable to a company with securities registered pursuant to Section 12 of the Exchange Act. In accordance therewith, we will file annual, quarterly and current reports, proxy statements, and other information with the SEC. All documents filed with the SEC are available for inspection and copying at the internet site of the SEC referred to above. We maintain a website at GroveInc.io. After the consummation of this offering, you may access our periodic reports, proxy statements and other information free of charge at this website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference and is not a part of this prospectus.
| 79 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
GROVE INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED JUNE 30, 2020 AND 2019
|
|
|
Page |
|
|
|
|
|
|
|
|
F-2 |
| |
|
|
|
|
|
|
Consolidated Financial Statements |
|
|
|
|
|
|
|
|
|
|
F-4 |
| |
|
|
|
|
|
|
|
F-5 |
| |
|
|
|
|
|
|
|
F-6 |
| |
|
|
|
|
|
|
|
F-7 |
| |
|
|
|
|
|
|
|
F-8 |
|
Interim Unaudited Condensed Consolidated Financial Statements
For the Quarterly Period Ended September 30, 2020
|
|
|
Page |
| |
|
|
|
|
| |
|
Condensed Consolidated Balance Sheets as of September 30, 2020 (Unaudited) and June 30, 2020 |
|
|
F-26 |
|
|
|
|
|
|
|
|
|
|
F-27 |
| |
|
|
|
|
|
|
|
|
|
F-28 |
| |
|
|
|
|
|
|
|
|
|
F-29 |
| |
|
|
|
|
|
|
|
Notes to the Unaudited Condensed Consolidated Financial Statements |
|
|
F-30 |
|
INFUSIONZ LLC
TABLE OF CONTENTS
|
|
Page |
| |
|
|
|
|
|
|
|
F-40 |
| |
|
|
|
|
|
|
Financial Statements |
|
|
|
|
|
|
|
|
|
|
F-41 |
| |
|
|
|
|
|
|
|
F-42 |
| |
|
|
|
|
|
|
|
F-43 |
| |
|
|
|
|
|
|
|
F-44 |
| |
|
|
|
|
|
|
|
F-45 |
|
| F-1 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Grove, Inc.:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Grove, Inc. (“the Company”) as of June 30, 2020, and the related consolidated statements of operations, stockholders’ equity, and cash flows for the year ended June 30, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Grove, Inc. as of June 30, 2020, and the results of its operations and its cash flows for the year ended June 30, 2020, in conformity with ●●accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
/s/ B F Borgers CPA PC
We have served as the Company’s auditor since 2020.
Lakewood, Colorado
February 10, 2021
| F-2 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Grove, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Grove, Inc. (the Company) as of June 30, 2019, and the related consolidated statements of operations and changes in members’ interest, and consolidated statements of cash flows for the year then ended and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2019, and the results of its operations and its cash flows for the year then ended June 30, 2019, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ RBSM LLP
We have served as the Company’s auditor since 2019.
Henderson, Nevada
June 25, 2020
| F-3 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
GROVE, INC.
Consolidated Balance Sheets
As of June 30, 2020 and 2019
(In U.S. Dollars, except share data or otherwise stated)
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
ASSETS |
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
| ||
|
Cash |
|
$ | 887,517 |
|
|
$ | 3,697,432 |
|
|
Accounts receivable, net (allowance for doubtful accounts was $10,000 and $0, respectively) |
|
|
165,147 |
|
|
|
127,722 |
|
|
Other receivables |
|
|
72,000 |
|
|
|
20,000 |
|
|
Inventory |
|
|
1,448,448 |
|
|
|
1,138,064 |
|
|
Prepaid expenses |
|
|
76,562 |
|
|
|
131,976 |
|
|
Total current assets |
|
|
2,649,674 |
|
|
|
5,115,194 |
|
|
|
|
|
|
|
|
|
|
|
|
Property and Equipment, net |
|
|
1,687,273 |
|
|
|
262,015 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,240,260 |
|
|
|
1,633,738 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
493,095 |
|
|
Deposit |
|
|
37,068 |
|
|
|
- |
|
|
Right-of-use asset |
|
|
294,835 |
|
|
|
- |
|
|
Total other assets |
|
|
3,752,531 |
|
|
|
2,388,848 |
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ | 6,402,205 |
|
|
$ | 7,504,042 |
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ | 484,333 |
|
|
$ | 271,062 |
|
|
Deferred revenue |
|
|
473,320 |
|
|
|
255,633 |
|
|
Accrued liabilities related to acquisition |
|
|
- |
|
|
|
287,528 |
|
|
Accrued liabilities |
|
|
417,063 |
|
|
|
293,336 |
|
|
Current portion of notes payable |
|
|
183,595 |
|
|
|
- |
|
|
Convertible note payable |
|
|
1,500,000 |
|
|
|
- |
|
|
Current portion of operating lease payable |
|
|
461,123 |
|
|
|
- |
|
|
Total current liabilities |
|
|
3,519,434 |
|
|
|
1,107,559 |
|
|
|
|
|
|
|
|
|
|
|
|
Notes payable, net of current portion |
|
|
365,350 |
|
|
|
- |
|
|
Operating lease payable, net of current portion |
|
|
338,040 |
|
|
|
- |
|
|
Total long-term liabilities |
|
|
703,390 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 1,000,000 shares authorized, 0 and 500,000 shares issued and outstanding, respectively |
|
|
- |
|
|
|
500 |
|
|
Common stock, $0.001 par value, 100,000,000 shares authorized, and 18,400,000 and 17,376,470 shares issued and outstanding, respectively |
|
|
18,400 |
|
|
|
17,376 |
|
|
Additional paid in capital |
|
|
7,306,164 |
|
|
|
6,438,918 |
|
|
Accumulated deficit |
|
|
(7,098,984 | ) |
|
|
(1,715,311 | ) |
|
Total stockholders' equity |
|
|
225,580 |
|
|
|
4,741,483 |
|
|
Non-controlling interest in subsidiary |
|
|
1,953,801 |
|
|
|
1,655,000 |
|
|
Total equity |
|
|
2,179,381 |
|
|
|
6,396,483 |
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders' equity |
|
$ | 6,402,205 |
|
|
$ | 7,504,042 |
|
| F-4 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Consolidated Statements of Operations
Years Ended June 30, 2020 and 2019
(In U.S. Dollars, except share data or otherwise stated)
|
|
|
Year Ended June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
Revenue |
|
|
|
|
|
| ||
|
Product revenue |
|
|
6,159,013 |
|
|
|
1,428,302 |
|
|
Trade show revenue |
|
|
1,253,847 |
|
|
|
779,750 |
|
|
|
|
|
7,412,860 |
|
|
|
2,208,052 |
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
4,280,909 |
|
|
|
945,756 |
|
|
Trade show cost |
|
|
561,988 |
|
|
|
226,099 |
|
|
|
|
|
4,842,897 |
|
|
|
1,171,855 |
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
2,569,963 |
|
|
|
1,036,197 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
1,370,964 |
|
|
|
162,066 |
|
|
General and administrative expenses |
|
|
5,272,997 |
|
|
|
1,227,361 |
|
|
Professional fees |
|
|
764,332 |
|
|
|
235,823 |
|
|
|
|
|
7,408,293 |
|
|
|
1,625,250 |
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(4,838,330 | ) |
|
|
(589,053 | ) |
|
|
|
|
|
|
|
|
|
|
|
Other expense (income), net |
|
|
|
|
|
|
|
|
|
Gain on the sale of assets |
|
|
(180,211 | ) |
|
|
- |
|
|
Other expense (income), net |
|
|
- |
|
|
|
1,055 |
|
|
Impairment of cancelled lease expense |
|
|
588,347 |
|
|
|
- |
|
|
Interest expense (income), net |
|
|
138,406 |
|
|
|
(3,068 | ) |
|
Other expense (income), net |
|
|
546,542 |
|
|
|
(2,013 | ) |
|
|
|
|
|
|
|
|
|
|
|
Net loss before income tax |
|
|
(5,384,872 | ) |
|
|
(587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
Income tax expense |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
(5,384,872 | ) |
|
|
(587,040 | ) |
|
Net income (loss) attributable to noncontrolling interest |
|
|
(1,199 | ) |
|
|
- |
|
|
Net loss attributable to Grove, Inc. |
|
|
(5,383,673 | ) |
|
|
(587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
Earnings per share attributable to Grove, Inc. common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
$ | (0.30 | ) |
|
$ | (0.05 | ) |
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding |
|
|
18,174,735 |
|
|
|
12,647,631 |
|
| F-5 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
GROVE, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(In U.S. Dollars, except share data or otherwise stated)
|
|
|
Preferred Stock |
|
|
Preferred Stock |
|
|
Common Stock |
|
|
Common Stock |
|
|
Additional Paid |
|
|
Accumulated |
|
|
Non-controlling |
|
|
Shareholders' |
| ||||||||
|
|
|
Shares |
|
|
Par |
|
|
Shares |
|
|
Par |
|
|
In Capital |
|
|
Deficit |
|
|
Interest |
|
|
Equity |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Balance, June 30, 2018 |
|
|
- |
|
|
$ | - |
|
|
|
11,700,000 |
|
|
$ | 11,700 |
|
|
$ | 1,034,080 |
|
|
$ | (1,128,271 | ) |
|
$ | - |
|
|
$ | (82,491 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trunano Labs, Inc. subsidiary stock issued for cash |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,655,000 |
|
|
|
1,655,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued for cash |
|
|
500,000 |
|
|
|
500 |
|
|
|
5,676,470 |
|
|
|
5,676 |
|
|
|
4,663,824 |
|
|
|
- |
|
|
|
- |
|
|
|
4,670,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholder contribution |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
136,457 |
|
|
|
- |
|
|
|
- |
|
|
|
136,457 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
604,557 |
|
|
|
- |
|
|
|
- |
|
|
|
604,557 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(587,040 | ) |
|
|
- |
|
|
|
(587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2019 |
|
|
500,000 |
|
|
$ | 500 |
|
|
|
17,376,470 |
|
|
$ | 17,376 |
|
|
$ | 6,438,918 |
|
|
$ | (1,715,311 | ) |
|
$ | 1,655,000 |
|
|
$ | 6,396,483 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2019 |
|
|
500,000 |
|
|
$ | 500 |
|
|
|
17,376,470 |
|
|
$ | 17,376 |
|
|
$ | 6,438,918 |
|
|
$ | (1,715,311 | ) |
|
$ | 1,655,000 |
|
|
$ | 6,396,483 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued for cash |
|
|
|
|
|
|
|
|
|
|
523,530 |
|
|
|
524 |
|
|
|
444,476 |
|
|
|
- |
|
|
|
- |
|
|
|
445,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of preferred stock |
|
|
(500,000 | ) |
|
|
(500 | ) |
|
|
500,000 |
|
|
|
500 |
|
|
|
50,000 |
|
|
|
- |
|
|
|
- |
|
|
|
50,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trunano Labs, Inc. subsidiary stock issued for cash |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
300,000 |
|
|
|
300,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
372,770 |
|
|
|
- |
|
|
|
- |
|
|
|
372,770 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5,383,673 | ) |
|
|
(1,199 | ) |
|
|
(5,384,872 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2020 |
|
|
- |
|
|
$ | - |
|
|
|
18,400,000 |
|
|
$ | 18,400 |
|
|
$ | 7,306,164 |
|
|
$ | (7,098,984 | ) |
|
$ | 1,953,801 |
|
|
$ | 2,179,381 |
|
| F-6 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Consolidated Statements of Cash Flows
For the Year’s Ended June 30, 2020 and 2019
(In U.S. Dollars, except share data or otherwise stated)
|
|
|
Year Ended June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Cash flows from operating activities |
|
|
|
|
|
| ||
|
Net loss |
|
$ | (5,383,673 | ) |
|
$ | (587,040 | ) |
|
Adjustments to reconcile net loss to net cash used by operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
611,346 |
|
|
|
67,568 |
|
|
Impairment loss on leased asset |
|
|
588,347 |
|
|
|
- |
|
|
Provision for doubtful accounts and bad debt expense |
|
|
160,740 |
|
|
|
11,779 |
|
|
Gain on sale of equipment |
|
|
(180,211 | ) |
|
|
- |
|
|
Stock based compensation |
|
|
372,770 |
|
|
|
604,557 |
|
|
Changes in assets and liabilities (net of amounts acquired): |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(95,665 | ) |
|
|
(54,465 | ) |
|
Other receivables |
|
|
(154,500 | ) |
|
|
106,376 |
|
|
Inventory |
|
|
(310,384 | ) |
|
|
(620,371 | ) |
|
Prepaid expenses and other assets |
|
|
18,346 |
|
|
|
(118,472 | ) |
|
Accounts payable and accrued liabilities |
|
|
277,979 |
|
|
|
(665,658 | ) |
|
Accrued liabilities related to acquisition |
|
|
(287,528 | ) |
|
|
(108,180 | ) |
|
Deferred revenue |
|
|
217,687 |
|
|
|
169,467 |
|
|
Net cash used by operating activities |
|
|
(4,164,746 | ) |
|
|
(1,194,439 | ) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Proceeds from sale of property and equipment |
|
|
466,113 |
|
|
|
- |
|
|
Acquisition of property and equipment |
|
|
(1,929,028 | ) |
|
|
(219,448 | ) |
|
Acquisition of HAVZ, net of cash |
|
|
- |
|
|
|
(1,451,788 | ) |
|
Net cash used in investing activities |
|
|
(1,462,915 | ) |
|
|
(1,671,236 | ) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net |
|
|
420,000 |
|
|
|
4,670,000 |
|
|
Proceeds from issuance of common stock for conversion of preferred stock |
|
|
50,000 |
|
|
|
- |
|
|
Proceeds from issuance of non-controlling interest, net |
|
|
298,801 |
|
|
|
1,655,000 |
|
|
Proceeds from issuance of notes payable |
|
|
2,048,945 |
|
|
|
- |
|
|
Shareholder contributions, net |
|
|
- |
|
|
|
136,457 |
|
|
Net cash provided by financing activities |
|
|
2,817,746 |
|
|
|
6,461,457 |
|
|
|
|
|
|
|
|
|
|
|
|
Net (decrease) increase in cash |
|
|
(2,809,915 | ) |
|
|
3,595,782 |
|
|
Cash, beginning of period |
|
|
3,697,432 |
|
|
|
101,650 |
|
|
Cash, end of period |
|
$ | 887,517 |
|
|
$ | 3,697,432 |
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental cash flow disclosures |
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ | 2,521 |
|
|
$ | - |
|
|
Income tax paid |
|
$ | - |
|
|
$ | - |
|
|
Non-cash financing activities |
|
|
|
|
|
|
|
|
|
Stock issuance for payroll accrual |
|
$ | 25,000 |
|
|
$ | - |
|
| F-7 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Notes to Consolidated Financial Statements
Years Ended June 30, 2020 and 2019
Note 1. Background Information
We are in the business of developing, producing, marketing, and selling raw materials, white label products and end consumer products containing the hemp plant extract, Cannabidiol (“CBD”). We sell to numerous consumer markets including the nutraceutical, beauty care, pet care and functional food sectors. We seek to take advantage of an emerging worldwide trend to re-energize the production of industrial hemp and to foster its many uses for consumers.
In addition, we are an operator of an annual tradeshow in the United States related to the CBD industry. The trade show business is naturally seasonal. The Company only has one trade show, CBD.IO, which is held in November each year. Because event revenue is recognized when a particular event is held, the Company experiences fluctuations in quarterly revenue based on the completion of the trade show event. The seasonality of the business is typical of the trade show industry.
Grove, Inc. (the “Company”) is a Nevada Corporation and has six wholly owned subsidiaries, Cresco Management, LLC, a California limited liability company, Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, and One Hit Wonder Holdings, LLC, a California limited liability company, and SWCH, LLC, a Delaware limited liability company. In addition, the Company has the controlling interest (79.4%) in Trunano Labs, Inc., a Nevada corporation.
On July 1, 2020, the noncontrolling shareholders of the Company’s subsidiary, Trunano Labs, Inc., converted 1,761,261 shares of Trunano Labs, Inc. stock, representing all the outstanding stock by minority interest holders, into 2,300,000 shares of Grove, Inc. common stock. As of July 1, 2020, Trunano Labs, Inc. is a wholly owned subsidiary of Grove, Inc.
On May 31, 2019, the Company purchased Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale, a California limited liability company, and One Hit Wonder Holdings, LLC, a California limited liability company, collectively known as “HAVZ Consolidated” out of bankruptcy.
In December of 2018, HAVZ Consolidated filed voluntary petitions for relief under Chapter 11 (Chapter 11 Proceedings) of the U.S. Bankruptcy Code in the U.S. Bankruptcy Court for the District of Nevada. On May 31, 2019, in connection with the Section 363 Sale (“Buying Assets from a Bankrupt Company”), these four entities, HAVZ Consolidated, were purchased by the Company for a payment of $2,100,000 to the creditors of HAVZ Consolidated. The accompanying consolidated financial statements include the financial statements of HAVZ Consolidated for the period of June 1, 2019 to June 30, 2020.
Liquidity and Going Concern
The Company experienced significant net losses in fiscal 2020 and fiscal 2019. Management has implemented a strategy which includes cost reductions and consolidation of certain operating activities to gain efficiencies as well as identifying strategic acquisitions, financed primarily through a combination of the issuance of equity and debt, to improve the overall profitability and cash flows of the Company. As of April 1, 2020, the Company ceased production operations in California and has consolidated operations into a single location in Nevada. These factors raise substantial doubts about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event that the Company cannot continue as a going concern.
As of June 30, 2020, the Company had cash of approximately $887,517 and a working capital deficit of approximately $869,760.
| F-8 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The ability to continue as a going concern is dependent upon the Company generating profitable operations in the future and/or obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. Management intends to finance operating costs over the next twelve months from the date of the issuance of these consolidated financial statements with existing cash on hand and/or the private placement of common stock. There is, however, no assurance that the Company will be able to raise any additional capital through any type of offering on terms acceptable to the Company, as existing cash on hand will be insufficient to finance operations over the next twelve months.
Basis of Presentation and Principles of Consolidation
The Company’s consolidated financial statements are prepared in accordance with accounting principles general accepted in the United States (“GAAP”). The consolidated financial statements include the accounts of all subsidiaries in which the Company holds a controlling financial interest as of the June 30, 2020 and 2019.
The consolidated financial statements include the accounts of the Company, Trunano Labs, Inc.,(for which the Company owns 79.4%) and its wholly owned subsidiaries; Steam Distribution, LLC, One Hit Wonder, Inc., Havz, LLC, d/b/a Steam Wholesale, One Hit Wonder Holdings, SWCH, LLC and Cresco Management LLC. All intercompany accounts and transactions have been eliminated in consolidation. As of the date of this report, Trunano Labs, Inc. and One Hit Wonder Holdings, LLC had no operations.
For the years ended June 30, 2020 and June 30, 2019 the financial statements of Grove Inc. are reported as consolidated entities, including Cresco Management LLC, HAVZ Consolidated and SWCH, LLC and for the year ended June 30, 2020, the financial statements of Grove Inc. report the activity of Cresco Management, LLC and SWCH, LLC for the 12 months ended June 30, 2019 and one month of HAVZ Consolidated since the acquisition of the four entities of HAVZ Consolidated was May 31, 2019.
Note 2. Significant Accounting Policies
The significant accounting policies followed are:
Use of Estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates underlying the Company’s reported financial position and results of operations include the allowance for doubtful accounts, useful lives of property and equipment, impairment of long lived assets, inventory valuation, fair value of stock-based compensation and valuation allowance on deferred tax assets.
Cash and Cash Equivalents - The Company considers all highly liquid debt instruments with a maturity of three months or less to be cash equivalents. Cash and cash equivalents are maintained at financial institutions and, at times, balances may exceed federally insured limits. The Company has never experienced any losses related to these balances.
Accounts Receivable - The Company regularly reviews accounts receivable for any bad debts based on an analysis of the Company’s collection experience, customer credit worthiness and current economic trends. After all attempts to collect a receivable have failed, the receivable is written off against the allowance. Based on management’s review of accounts receivable, the Company recorded $10,000 and $0 as allowance for doubtful accounts at June 30, 2020 and 2019, respectively. The Company had bad debt expense of $160,740 and $11,779 for the years ended June 30, 2020 and 2019, respectively, including write-offs of accounts receivables $150,740 and $11,779, for the years ended June 30, 2020 and 2019, respectively.
Inventory - Inventory consists of raw materials, work-in-process and finished goods and is stated at the lower of cost or net realizable value, cost is determined by the weighted average moving cost inventory method. Net realizable value is determined, with appropriate consideration given to obsolescence, excessive levels, deterioration, and other factors.
| F-9 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Property and Equipment - Property and equipment is recorded at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets ranging from 3 to 7 years. Leasehold improvements are amortized over the shorter of their estimated useful lives of 5 years or the related lease term. Gains and losses upon disposition are reflected in the Statement of Operations in the period of disposition. Maintenance and repair expenditures are charged to expense as incurred. The Company disposed of some equipment during 2020 which resulted in a gain on the sale. There were no dispositions of property and equipment in 2019.
Business Combinations -The Company accounts for its business combinations using the acquisition method of accounting. The cost of an acquisition is measured as the aggregate of the acquisition date fair values of the assets transferred and liabilities assumed by the Company to the sellers cash consideration and equity instruments issued. Transaction costs directly attributable to the acquisition are expensed as incurred. The excess of (i) the total costs of acquisition over (ii) the fair value of the identifiable net assets of the acquiree is recorded as identifiable intangible assets and goodwill.
Impairment of Long-lived Assets - Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the asset may not be recoverable. The Company periodically evaluates whether events and circumstances have occurred that indicate possible impairment. When impairment indicators exist, the Company estimates the future undiscounted net cash flows of the related asset or asset group over the remaining life in measuring whether or not the asset values are recoverable. The Company did not recognize impairment on its long-lived assets during the years ended June 30, 2020 or 2019.
Revenue Recognition - The Company has adopted the new revenue recognition guidelines in accordance with ASC 606, Revenue from Contracts with Customers (ASC 606), commencing from the period under this report. The Company analyzes its contracts to assess that they are within the scope and in accordance with ASC 606. In determining the appropriate amount of revenue to be recognized as the Company fulfills its obligations under each of its agreements, whether for goods and services or licensing, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. The Company acts as a principal in its revenue transactions as the Company is the primary obligor in the transactions. Generally, the Company recognizes revenue for its products upon shipment to customers, provided no significant obligations remain and collection is probable.
Product Revenue - Most of the Company’s revenue contracts are from domestic sales and represent a single performance obligation related to the fulfillment of customer orders for the purchase of its products. Net sales reflect the transaction prices for these contracts based on the Company’s selling list price, which is then reduced by estimated costs for trade promotional programs, consumer incentives, and allowances and discounts used to incentivize sales growth and build brand awareness.
The Company recognizes revenue at the point in time that control of the ordered product is transferred to the customer, which is upon shipment to the customer or other customer-designated delivery point. Taxes collected from customers that are remitted to governmental agencies are accounted for on a net basis and not included as revenue.
The Company does not accept sales returns from wholesale customers, as the products are pre-approved prior to production and shipment. E-Commerce product returns must be completed within 45 days of the date of purchase. The Company does not accrue for estimated sales returns as historical sales returns have been minimal. The Company records deferred revenues when cash payments are received or due in advance of performance, including amounts which are refundable. Substantially all the deferred revenue as of June 30, 2019 was recognized as revenue in the year ended June 30, 2020.
Shipping and handling fees billed to customers are included in revenue. Shipping and handling fees associated with freight are generally included in cost of revenue.
Trade Show Revenue - A significant portion of the Company’s annual revenue is generated from the production of a single trade show. The revenue includes booth space sales, registration fees and sponsorship fees. The Company recognizes revenue upon completion of the CBD.IO trade show. Amounts invoiced prior to the completion of the trade show are recorded as deferred revenues in the consolidated balance sheets until the completion of the event. As of June 30, 2020, and 2019, the Company had no deferred revenue related to trade show business.
| F-10 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Loyalty Program - The Company grants customers loyalty points for each purchase on the website and at the time of the sale, accrues the estimated cost related to fulfilling the future purchase in accrued liabilities. When the points are redeemed, the Company does not recognize any revenue related to the purchase and reduces the accrued liability related to the cost of the purchase.
Advertising - The Company supports its products with advertising to build brand awareness of the Company’s various products in addition to other marketing programs executed by the Company’s marketing team. The Company believes the continual investment in advertising is critical to the development and sale of its CBD branded products. Advertising costs of $165,640 and $64,985 were expensed as incurred during the years ended June 30, 2020 and 2019, respectively.
Stock Based Compensation - The Company recognizes all share-based payments to employees, including grants of employee stock options, as compensation expense in the financial statements based on their fair values. That expense will be recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period) or immediately if the share-based payments vest immediately.
Non-employee Stock-based Payments - The Company’s accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows the provisions of ASC 2018-07, which simplifies the accounting for non-employee share-based payment transactions. The amendments specify that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. Stock-based payments related to non-employees is accounted for based on the fair value of the related stock or options or the fair value of the services, whichever is more readily determinable. The measurement date for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance by the consultant or vendor is reached or (ii) the date at which the consultant or vendor’s performance is complete. In the case of equity instruments issued to consultants, the fair value of the equity instrument is recognized over the term of the consulting agreement.
Fair Value Measurements - The Company accounts for financial instruments in accordance with FASB Accounting Standards Codification (ASC) 820 “Fair value Measurement and Disclosures” (ASC 820). ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs).
The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below:
|
• |
Level 1 - Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. | |
|
• |
Level 2 - Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly, including quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability (e.g. interest rates); and inputs that are derived principally from or corroborated by observable market data by correlation or other means. | |
|
• |
Level 3 - Inputs that are both significant to the fair value measurement and unobservable. |
| F-11 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The estimated fair value of certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, deferred revenue and debt are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
Leases - The Company determines if a contract contains a lease at inception. US GAAP requires that the Company’s leases be evaluated and classified as operating or finance leases for financial reporting purposes. The classification evaluation begins at the commencement date and the lease term used in the evaluation includes the non-cancellable period for which the Company has the right to use the underlying asset, together with renewal option periods when the exercise of the renewal option is reasonably certain and failure to exercise such option will result in an economic penalty. All of the Company’s real estate leases are classified as operating leases.
Most real estate leases include one or more options to renew, with renewal terms that generally can extend the lease term for an additional two years. The exercise of lease renewal options is at the Company’s discretion. The Company evaluates renewal options at lease inception and on an ongoing basis and includes renewal options that it is reasonably certain to exercise in its expected lease terms when classifying leases and measuring lease liabilities. Lease agreements generally do not require material variable lease payments, residual value guarantees or restrictive covenants.
The Company’s leases generally do not provide an implicit rate, and therefore the Company uses its incremental borrowing rate as the discount rate when measuring operating lease liabilities. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease within a particular currency environment. The Company used incremental borrowing rates as of July 1, 2019 for operating leases that commenced prior to that date.
Income Taxes - Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due plus deferred taxes resulting from temporary differences. Such temporary differences result from differences in the carrying value of assets and liabilities for tax and financial reporting purposes. The deferred tax assets and liabilities represent the future tax consequences of those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
The Company identifies and evaluates uncertain tax positions, if any, and recognizes the impact of uncertain tax positions for which there is a less than more-likely-than-not probability of the position being upheld when reviewed by the relevant taxing authority. Such positions are deemed to be unrecognized tax benefits and a corresponding liability is established on the balance sheet. The Company has not recognized a liability for uncertain tax positions. If there were an unrecognized tax benefit, the Company would recognize interest accrued related to unrecognized tax benefits in interest expense and penalties in operating expenses.
One Hit Wonder, Inc. has elected S Corporation status for federal income tax and California corporation business tax purposes, Steam Distribution, LLC, Havz, LLC and One Hit Wonder Holdings, LLC elected partnership status for federal income tax and California corporation business tax purposes. Under these elections, the Company is not a taxpaying entity for federal and state income tax purposes and, accordingly, no provision has been made for such income taxes, except for a minimum state corporate business tax. The stockholders’ allocable share of the Company’s income or loss is reportable on his or her income tax return through May 31, 2019. These entities under Grove, Inc. are tax paying entities the period from June 1, 2019 to June 30, 2019 remains open and subject to examination by the Internal Revenue Service.
Cresco Management, LLC and SWCH, LLC elected partnership status for federal income tax and California and Delaware corporation business tax purposes, respectively. Under these elections, these Subsidiaries are not a taxpaying entity for federal and state income tax purposes and, accordingly, no provision has been made for such income taxes, except for a minimum state corporate business tax through December 31, 2018. The stockholders’ allocable share of the Company’s income or loss is reportable on his or her income tax return through December 31, 2018. The Company’s 2019 through 2020 tax years remain open and subject to examination by the Internal Revenue Service. Grove had no operations through December 31, 2018. On January 1, 2019 Cresco Management LLC and SWCH LLC were contributed to Grove, Inc. in a non-taxable transaction. Grove Inc. consolidated from 2018 to current. The first consolidated tax return for all entities was filed for the tax year December 31, 2019.
| F-12 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. The Company has not recognized any deferred tax asset or liabilities as of June 30, 2020 has a full valuation allowance for any possible future tax benefit from a net operating loss.
ASC Topic 740 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. There are no material uncertain tax positions at June 30, 2020.
On December 22, 2017, the U.S. government enacted the Tax Act, which made significant changes to the Internal Revenue Code of 1986, as amended, including, but not limited to, reducing the U.S. corporate statutory tax rate and the net operating loss incurred after December 31, 2017 can be carried forward indefinitely and the two year net operating loss carried back was eliminated (prohibited).
Earnings (loss) per Share - Basic earnings (loss) per share is computed by dividing net income (loss) attributable to common stockholders by the weighted average common shares outstanding for the period. Diluted income (loss) per share is computed giving effect to all potentially dilutive common shares. Potentially dilutive common shares may consist of incremental shares issuable upon the exercise of stock options and warrants and upon the conversion of notes. In periods in which a net loss has been incurred, all potentially dilutive common shares are considered anti-dilutive and thus are excluded from the calculation.
Deferred Revenue - The Company records deposits as deferred revenue when a customer pays in advance of shipping the product. Once the product is shipped, the deposit is recorded as revenue and the related commissions are paid. All products were shipped related to deposits in deferred revenue, in less than one year.
The Company recognizes revenue upon completion of the CBD.IO trade show. Amounts invoiced prior to the completion of the trade show are recorded as deferred revenues in the consolidated balance sheets until the completion of the event.
Non-controlling Interests in Consolidated Financial Statements - In December 2007, the FASB issued ASC 810-10-65, “Non-controlling Interests in consolidated Financial Statements”. This ASC clarifies that a non-controlling (minority) interest in subsidiaries is an ownership interest in the entity that should be reported as equity in the consolidated financial statements. It also requires consolidated net income to include the amounts attributable to both the parent and non-controlling interest, with disclosure on the face of the consolidated income statement of the amounts attributed to the parent and to the non-controlling interest. In accordance with ASC 810-10-45-21, those losses attributable to the parent and the non-controlling interest in subsidiaries may exceed their interests in the subsidiary’s equity. The excess and any further losses attributable to the parent and the non-controlling interest shall be attributed to those interests even if that attribution results in a deficit non-controlling interest balance.
Operating Segments - The Company’s financial reporting is organized into two segments: products and trade shows for revenue and cost of revenue. The Company’s internal reporting for product sales is organized into three channels of distribution: Grove, Inc. branded products, manufacturing of products to be sold under customers brands and white label products that are sold under customer brands. These product sales are aggregated and viewed by management as one reportable segment due to their similar economic characteristics, products, production, distribution processes and regulatory environment.
The Company does not allocate or track certain sales, general and administrative expenses to individual reportable segments.
| F-13 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Reclassifications - Certain reclassifications have been made to the financial statements as of and for the year ended June 30, 2019 to conform to the presentation as of and for the year ended June 30, 2020.
Recent Accounting Pronouncements - There are new accounting pronouncements issued by the Financial Accounting Standards Board (“FASB”) which are not yet effective as follows:
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (“ASC 326”), authoritative guidance amending how entities will measure credit losses for most financial assets and certain other instruments that are not measured at fair value through net income. The guidance requires the application of a current expected credit loss model, which is a new impairment model based on expected losses. The new guidance is effective for interim and annual reporting periods beginning after December 15, 2022. The Company is currently evaluating the impact of the new guidance on its consolidated financial statements and related disclosures.
In August 2020, the FASB issued ASU 2020-06-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity, which simplifies the guidance for certain convertible debt instruments by removing the separation models for convertible debt with a cash conversion feature or convertible instruments with a beneficial conversion feature. As a result, convertible debt instruments will be reported as a single liability instrument with no separate accounting for embedded conversion features. Additionally, ASU 2020-06 requires the application of the if-converted method for calculating diluted earnings per share and the treasury stock method will be no longer available. The provisions of ASU 2020-06 are applicable for fiscal years beginning after December 15, 2021, with early adoption permitted no earlier than fiscal years beginning after December 15, 2020. The Company expects the primary impacts of this new standard will be to increase the carrying value of its Convertible Debt and reduce its reported interest expense. In addition, the Company will be required to use the if-converted method for calculating diluted earnings per share. The Company is currently evaluating the impact the adoption of this standard will have on its condensed consolidated financial statements.
No other recent accounting pronouncements were issued by FASB and the SEC that are believed by management to have a material impact on the Company’s present or future unaudited condensed consolidated financial statements.
Note 3. Acquisitions
On May 31, 2019, Grove, Inc. purchased four entities, Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; HAVZ, LLC, d/b/a Steam Wholesale, a California limited liability company, and One Hit Wonder Holdings, LLC, a California corporation, are collectively known as “HAVZ Consolidated”. HAVZ Consolidated was purchased for a payment of $2,100,000 to the creditors of HAVZ Consolidated.
HAVZ Consolidated is in the business of developing, producing, marketing, and selling raw materials, white label products and end consumer products containing the hemp plant extract, Cannabidiol (“CBD”). HAVZ Consolidated sells to numerous consumer markets including the nutraceutical, beauty care, pet care and functional food sectors. HAVZ Consolidated seeks to take advantage of an emerging worldwide trend to re-energize the production of industrial hemp and to foster its many uses for consumers.
The intangibles were recorded, based on the Company’s estimate of fair value, which consist primarily of customer lists and trade name with an estimated life of ten years and goodwill. Upon completion of an independent purchase price allocation and valuation, the allocation intangible assets were adjusted accordingly.
| F-14 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The assets and liabilities of HAVZ Consolidated are recorded at their respective fair values as of May 31, 2019, and the following table summarizes these values.
|
Purchase Price Allocation |
| |||
|
|
|
|
| |
|
Tangible Assets |
|
$ | 1,440,478 |
|
|
Intangible Assets |
|
|
1,665,815 |
|
|
Goodwill |
|
|
493,095 |
|
|
Liabilities Acquired |
|
|
(1,499,387 | ) |
|
Purchase Price |
|
$ | 2,100,000 |
|
The following unaudited pro forma combined financial information is based on the historical financial statements of the Company and HAVZ Consolidated, after giving effect to the Company’s acquisition of HAVZ Consolidated.
The following unaudited pro forma information does not purport to present what the Company’s actual results would have been had the acquisition occurred on July 1, 2018, nor is the financial information indicative of the results of future operations. The following table represents the unaudited consolidated pro forma results of operations for the year ended June 30, 2019 as if the acquisition occurred on July 1, 2018. Operating expenses have been increased for the amortization expense associated with the estimated fair value adjustment as of June 30, 2019 of expected definite lived intangible assets of approximately $385,000 per year.
|
Pro Forma, unaudited |
|
Year Ended June 30, |
| |
|
Net sales |
|
$ | 6,706,034 |
|
|
Cost of sales |
|
|
3,039,736 |
|
|
Operating expenses |
|
|
4,732,035 |
|
|
Net loss |
|
$ | (1,119,737 | ) |
|
Basic and dilutive income per common share |
|
$ | (0.09 | ) |
The Company’s consolidated financial statements for the year ended June 30, 2020 and 2019 include the actual results of HAVZ Consolidated since the date of acquisition. All of the operations of HAVZ Consolidated are included in the operations of the Company for the year ended June 30, 2020.
|
Revenue and net income for HAVZ for the year ended June 30, 2019 included in the statement of operations |
|
Revenue |
|
|
Net Loss |
| ||
|
|
|
|
|
|
|
| ||
|
HAVZ Consolidated |
|
$ | 428,379 |
|
|
$ | 32,639 |
|
Note 4. Inventory
Inventory consisted of the following:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Raw materials |
|
$ | 730,000 |
|
|
$ | 489,095 |
|
|
Finished goods |
|
|
718,448 |
|
|
|
648,969 |
|
|
Property and equipment, gross |
|
$ | 1,448,448 |
|
|
$ | 1,138,064 |
|
The Company writes-off the value of inventory deemed excessive or obsolete. As of June 30, 2020, and 2019, the Company did not deem that an inventory write-off was considered necessary.
| F-15 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Note 5. Property and Equipment
Property and equipment consist of the following:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Furniture and fixtures |
|
$ | 4,167 |
|
|
$ | 4,167 |
|
|
Computer equipment |
|
|
48,606 |
|
|
|
29,921 |
|
|
Manufacturing equipment |
|
|
45,692 |
|
|
|
30,293 |
|
|
Leasehold improvements |
|
|
1,787,200 |
|
|
|
199,916 |
|
|
Property and equipment, gross |
|
|
1,885,665 |
|
|
|
264,297 |
|
|
Less accumulated depreciation |
|
|
(198,392 | ) |
|
|
(2,282 | ) |
|
Property and equipment, net |
|
$ | 1,687,273 |
|
|
$ | 262,015 |
|
During the year ended June 30, 2020, the Company sold manufacturing equipment with a carrying value of approximately $289,789 for cash proceeds of $470,000 which resulting in a gain on the disposal of approximately $180,211.
Depreciation expense for the years ended June 30, 2020 and 2019 was $217,868 and $3,416, respectively.
Note 6. Intangible Assets
As of June 30, 2019
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Loss on Impairment of Intangible Assets |
|
|
Net Book Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Customer relationships |
|
$ | 1,199,260 |
|
|
$ | 24,301 |
|
|
$ | - |
|
|
$ | 1,174,959 |
|
|
Trade name |
|
|
466,555 |
|
|
|
7,776 |
|
|
|
- |
|
|
|
458,779 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ | 1,665,815 |
|
|
$ | 32,077 |
|
|
$ | - |
|
|
$ | 1,633,738 |
|
As of June 30, 2020
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Loss on Impairment of Intangible Assets |
|
|
Net Book Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Customer relationships |
|
$ | 1,199,260 |
|
|
$ | 324,467 |
|
|
$ | - |
|
|
$ | 874,793 |
|
|
Trade name |
|
|
466,555 |
|
|
|
101,088 |
|
|
|
- |
|
|
|
365,467 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ | 1,665,815 |
|
|
$ | 425,555 |
|
|
$ | - |
|
|
$ | 1,240,260 |
|
For the years ended June 30, 2020 and 2019, the Company amortized approximately $393,478 and $32,077, respectively, related to the customer list and trade name intangible asset. The customer list is being amortized on a straight-line basis over 4 years. The trade names are being amortized on a straight-line basis over 5 years.
|
Future amortization of intangible assets are as follows: |
|
|
|
|
|
|
|
|
|
|
|
June 30, 2021 |
|
$ | 384,908 |
|
|
June 30, 2022 |
|
|
384,908 |
|
|
June 30, 2023 |
|
|
384,908 |
|
|
June 30, 2024 |
|
|
85,536 |
|
|
|
|
$ | 1,240,260 |
|
| F-16 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Note 7. Operating Leases
During November 2019, the Company entered into a new lease for a Nevada facility that commenced on November 13, 2019 and recorded a right of use asset and corresponding lease liability. Lease expense was $151,978 for the year ended June 30, 2020.
During July 2019, the Company entered into a lease for a California facility that commenced on July 1, 2019 and recorded a right of use asset and corresponding lease liability. Lease expense was $194,163 for the year ended June 30, 2020. In March 2020, the Company consolidated operations to its Nevada facility and abandoned its manufacturing and sales facility in Costa Mesa, California. On March 31, 2020 there were 27 months remaining on the lease with an estimated cost of $23,800 per month. At June 30, 2020 the Company owed approximately $64,700 related to the adverse lease and management has estimated the expense related to this adverse lease to be approximately $588,347 as the undiscounted future liability of this lease and recorded an impairment loss of $558,918 related to the right of use of this asset.
For month to month leases and leases that expire in one year or less, lease expense for the year ended June 30, 2020 was $59,958.
The Company’s weighted average remaining lease term and weighted average discount rate for operating leases as of June 30, 2020 are:
|
Weighted average remaining lease term |
|
16 Months |
|
|
Weighted average incremental borrowing rate |
|
5.0 |
% |
For the year ended June 30, 2020, the components of lease expense, included in general and administrative expenses and interest expense in the consolidated statements of operations income, are as follows:
|
Operating lease cost: |
|
|
| |
|
Operating lease cost |
|
$ | 410,863 |
|
|
Amortization of ROU assets |
|
|
369,286 |
|
|
Impairment of cancelled lease |
|
|
588,347 |
|
|
Interest expense |
|
|
32,145 |
|
|
Total lease cost |
|
$ | 1,400,641 |
|
The table below reconciles the undiscounted future minimum lease payments (displayed by year and in the aggregate) under noncancelable operating leases with terms of more than one year to the total operating lease liabilities recognized in the consolidated balance sheet as of June 30, 2020:
|
2021 |
|
$ | 487,964 |
|
|
2022 |
|
|
353,950 |
|
|
Total undiscounted future minimum lease payments |
|
|
841,914 |
|
|
Less: Imputed interest |
|
|
(42,752 | ) |
|
Present value of operating lease obligation |
|
$ | 799,162 |
|
The Company has one leased facility that it is presently using, which is office, manufacturing, and warehouse space. The Company is responsible for real estate taxes, utilities, and repairs under the terms of certain of the operating leases. Therefore, all lease and non-lease components are combined and accounted for as single lease component.
| F-17 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Note 8. Accrued Liabilities
Accrued liabilities consist of the following:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Accrued expenses for loyalty program |
|
$ | 47,400 |
|
|
$ | 43,500 |
|
|
Accrued compensation |
|
|
195,399 |
|
|
|
94,241 |
|
|
Sales tax payable |
|
|
- |
|
|
|
5,595 |
|
|
Accrued interest |
|
|
93,543 |
|
|
|
- |
|
|
Accrued rent |
|
|
60,721 |
|
|
|
- |
|
|
Accrued travel expenses |
|
|
- |
|
|
|
150,000 |
|
|
Other accrued liabilities |
|
|
20,000 |
|
|
|
- |
|
|
|
|
$ | 417,063 |
|
|
$ | 293,336 |
|
Note 9. Convertible Promissory Notes and Notes Payable
During October of 2019, the Company entered into convertible promissory notes (Notes) for total proceeds of $1,500,000. The principal and interest of the Notes are payable in full at the maturity date of April 2021, if not previously converted. The Notes have an interest rate of 8%, total accrued interest is to be repaid at maturity, and are convertible into common stock if the Company enters a “Financing” arrangement which results in the Company’s common stock becoming listed or trading. The conversion rate would be equal to the price of the Company’s common stock sold in the “Financing”.
On April 28, 2020, the Company entered into a Paycheck Protection Program loan for $398,945 in connection with COVID-19. The promissory note has a fixed payment schedule, commencing seven months following the funding of the note and consisting of seventeen monthly payments of principal and interest, with the principal component of each payment based upon the level of amortization of principal over a two year period from the funding date. A final payment for the unpaid principal and accrued interest will be payable no later than April 28, 2022. The note bears interest at a rate of 1.00% per annum and is deferred for the first six months of the loan. Certain portions of the loan may qualify for loan forgiveness based on the terms of the program.
On June 3, 2020, the Company entered into a loan for $150,000 with the Small Business Administration. The promissory note has a fixed payment schedule commencing on June 3, 2021, consisting of principal and interest payments of $731 monthly. The balance of the principal and interest will payable thirty years from the date of the promissory note. The note bears interest at a rate of 3.75% per annum. The Small Business Administration has filed a UCC Financing Statement on this loan confirming it is collateralized by any and all tangible and intangible properties of the Company.
Convertible promissory notes and notes payable outstanding as of June 30, 2020 are summarized below:
|
|
|
Maturity Date |
|
June 30, 2020 |
| |
|
8% $1,500,000 Convertible Promissory Notes |
|
April 2021 |
|
$ | 1,500,000 |
|
|
3.75% $150,000 Note Payable |
|
June 2050 |
|
|
150,000 |
|
|
1% $398,945 Note Payable |
|
April 2022 |
|
|
398,945 |
|
|
Total notes payable |
|
|
|
|
2,048,945 |
|
|
Less current portion of notes payable |
|
|
|
|
1,683,595 |
|
|
Notes payable, less current portion |
|
|
|
$ | 365,350 |
|
| F-18 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
Future payments on convertible promissory notes and notes payable are as follows: |
|
|
| |
|
|
|
|
| |
|
June 30, 2021 |
|
$ | 1,683,595 |
|
|
June 30, 2022 |
|
|
224,853 |
|
|
June 30, 2023 |
|
|
8,772 |
|
|
June 30, 2024 |
|
|
8,772 |
|
|
June 30, 2025 |
|
|
8,772 |
|
|
Thereafter |
|
|
114,181 |
|
|
|
|
$ | 2,048,945 |
|
Note 10. Related Party Transactions
For the year ended June 30, 2019, the Company contracted with Mr. Good Vape, Inc. for product sales of approximately $1,609, recorded in product costs. Mr. Good Vape, Inc. is partially owned by members of management.
For the year ended June 30, 2019, the Company contracted with Insite Web Development LLC for consulting services for $5,000, recorded in selling, general and administrative expenses. Insite Web Development LLC is partially owned by members of management.
For the year ended June 30, 2020 and June 30, 2019, the Company leased the Las Vegas warehouse from a shareholder for $22,071 per month. This lease ended December 31, 2019 and there were no further liabilities related to this lease. The owner of the warehouse is also related to one of the members of management.
The above related party transactions are not necessarily indicative of the amounts and terms that would have been incurred had comparable transactions been entered into with independent parties.
Note 11. Equity Transactions
Preferred Stock
The Company’s Board of Directors has authorized 1,000,000 shares of preferred stock with a par value of $0.001 and issued 500,000 shares of preferred stock. This preferred stock is convertible into a single share of common stock at a price of $0.05 per share of preferred stock with additional terms and conditions determined by the Board of Directors. During the year ended June 30, 2020, an investor converted 500,000 shares of preferred stock into 500,000 shares of common stock for cash proceeds of $50,000.
Common Stock
During the year ended June 30, 2020, the Company issued 23,530 shares of common stock for cash of $20,000. The Company also issued 500,000 shares of common stock for the exercise of an option for $400,000 of cash proceeds and forgiveness of accrued payroll of $25,000 from the investor. Finally, the Company converted preferred stock into 500,000 shares of common stock for cash proceeds of $50,000. The Company issued a total of 1,023,530 shares of common stock for cash consideration of $470,000 and a reduction of accrued payable of $25,000.
The Company issued 5,676,470 shares of common stock and 500,000 shares of preferred stock for $0.85 per share for cash proceeds of $4,670,000, net of $150,000 offering costs, during the year ended June 30, 2019.
Trunano Labs, Inc. Common Stock
Trunano Labs, Inc. has 10,000,000 shares of common stock authorized with a par value of $0.001. As of June 30, 2020, Trunano Labs, Inc, had 7,261,261 issued and outstanding shares of common stock, of which 5,500,000 is owned by the Company. During the year ended June 30, 2019, Trunano Labs, Inc. issued 1,490,991 shares of common stock for cash proceeds of approximately $1,655,000. During the year ended June 30, 2020, Trunano Labs, Inc. issued 270,270 shares of common stock for cash proceeds of approximately $300,000. Primarily due to the decline in CBD isolate price, there were no operations during the year ended June 30, 2019 for Trunano Labs, Inc. During the year ended June 30, 2020, Trunano Labs, Inc. had a net loss of $5,850.
Shares of common stock of Trunano Labs, Inc. issued and outstanding as of:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Grove, Inc. |
|
|
5,500,000 |
|
|
|
5,500,000 |
|
|
Non-controlling interest |
|
|
1,761,261 |
|
|
|
1,490,991 |
|
|
Total shares issued and outstanding |
|
|
7,261,261 |
|
|
|
6,990,991 |
|
| F-19 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Note 12. Stock Based Compensation
The Company has established a Company an incentive plan, 2019 Equity Incentive Plan (the “2019 Plan”). The plan grants incentives to select persons who can make, are making and continue to make substantial contributions to the growth and success of the Company, to attract and retain the employment and services of such persons and to encourage and reward such contributions by providing these individuals with an opportunity to acquire or increase stock ownership in the Company through either the grant of options or restructured stock. The 2019 Plan is administered by the Compensation Committee or such other committee as is appointed by the Board of Directors pursuant to the 2019 Plan (the “Committee”). The Committee has full authority to administer and interpret the provisions of the 2019 Plan including, but not limited to, the authority to make all determinations with regard to the terms and conditions of an award made under the 2019 Plan. The maximum number of shares that may be granted under the 2019 Plan is 5,000,000.
The Board of Directors of the Company may from time to time, in its discretion grant to directors, officers, consultants and employees of the Company, non-transferable options to purchase common shares. The options are exercisable for a period of up to 10 years from the date of the grant.
The following table reflects the continuity of stock options for the year ended June 30, 2020:
A summary of stock option activity is as follows:
|
|
|
|
|
|
Weighted |
|
|
Average |
|
|
|
| ||||
|
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Aggregated |
| ||||
|
|
|
Options |
|
|
Exercise |
|
|
Contractual |
|
|
Intrinsic |
| ||||
|
|
|
Outstanding |
|
|
Price |
|
|
Life (Years) |
|
|
Value |
| ||||
|
Outstanding at June 30, 2018 |
|
|
- |
|
|
$ | - |
|
|
|
- |
|
|
$ | - |
|
|
Granted |
|
|
2,300,000 |
|
|
|
0.85 |
|
|
|
10 |
|
|
|
- |
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Expired |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Outstanding at June 30, 2019 |
|
|
2,300,000 |
|
|
$ | 0.85 |
|
|
|
10 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at June 30, 2019 (vested) |
|
|
1,108,333 |
|
|
|
0.85 |
|
|
|
10 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
Average |
|
|
|
|
| ||
|
|
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Aggregated |
| |||
|
|
|
Options |
|
|
Exercise |
|
|
Contractual |
|
|
Intrinsic |
| ||||
|
|
|
Outstanding |
|
|
Price |
|
|
Life (Years) |
|
|
Value |
| ||||
|
Outstanding at June 30, 2019 |
|
|
2,300,000 |
|
|
$ | 0.85 |
|
|
|
8.5 |
|
|
$ | - |
|
|
Granted |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercised |
|
|
(500,000 | ) |
|
$ | 0.85 |
|
|
|
- |
|
|
|
- |
|
|
Expired |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Outstanding at June 30, 2020 |
|
|
1,800,000 |
|
|
$ | 0.85 |
|
|
|
8.5 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at June 30, 2020 (vested) |
|
|
1,204,167 |
|
|
|
0.85 |
|
|
|
8.5 |
|
|
|
- |
|
The average fair value of stock options granted was estimated to be $0.57 per share for the period ended June 30, 2019.
| F-20 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Stock-based compensation expense attributable to stock options was approximately $372,770 and $604,557 for the years ended June 30, 2020 and 2019, respectively. As of June 30, 2020, there was approximately $341,706 unrecognized compensation expense related to unvested stock options outstanding, and the weighted average vesting period for those options was 1 years.
The value of each grant is estimated at the grant date using the Black-Scholes option model with the following assumptions for options granted during the year ended 2019. There were no options granted during the year ended June 30, 2020.
|
|
|
June 30, 2019 |
| |
|
Dividend rate |
|
|
- |
|
|
Risk free interest rate |
|
|
2.07 | % |
|
Expected term |
|
|
6.5 |
|
|
Expected volatility |
|
|
74 | % |
|
Grant date stock price |
|
$ | 0.85 |
|
The basis for the above assumptions are as follows: the dividend rate is based upon the Company’s history of dividends; the risk-free interest rate for periods within the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant; the expected term was calculated based on the Company’s historical pattern of options granted and the period of time they are expected to be outstanding; and expected volatility was calculated based upon historical trends in Charlotte’s Web Holdings, Inc. (CWBHF) stock prices for periods prior to the date the Company’s trading information was available. Management selected Charlotte’s Web Holdings, Inc. for it length of time as a publicly trading company and the similarities of the business and industry.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on historical experience of forfeitures, the Company estimated forfeitures at 0% for each of the years ended June 30, 2020 and 2019, respectively.
13. Income Taxes
One Hit Wonder, Inc. has elected S Corporation status for federal income tax and California corporation business tax purposes, Steam Distribution, LLC, Havz, LLC and One Hit Wonder Holdings, LLC elected partnership status for federal income tax and California corporation business tax purposes. Under these elections, the Company is not a taxpaying entity for federal and state income tax purposes and, accordingly, no provision has been made for such income taxes, except for a minimum state corporate business tax. The stockholders’ allocable share of the Company’s income or loss is reportable on his or her income tax return through May 31, 2019. These entities under Grove, Inc. are tax paying entities and the period from June 1, 2019 to June 30, 2019 remains open and subject to examination by the Internal Revenue Service.
Cresco Management, LLC and SWCH, LLC elected partnership status for federal income tax and California and Delaware corporation business tax purposes, respectively. Under these elections, these Subsidiaries are not a taxpaying entity for federal and state income tax purposes and, accordingly, no provision has been made for such income taxes, except for a minimum state corporate business tax through December 31, 2018. The stockholders’ allocable share of the Company’s income or loss is reportable on his or her income tax return through December 31, 2018. The Company’s 2019 through 2020 tax years remain open and subject to examination by the Internal Revenue Service. Grove had no operations through December 31, 2018. On January 1, 2019 Cresco Management LLC and SWCH, LLC were contributed to Grove, Inc. in a non-taxable transaction. Grove, Inc. consolidated from 2018 to current. The first consolidated tax return for all entities was filed for the tax year December 31, 2019.
| F-21 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The components of the provision for income taxes are as follows:
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
Current tax provision |
|
$ | - |
|
|
$ | - |
|
|
Deferred tax provision |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
$ | - |
|
|
$ | - |
|
The differences between income taxes calculated at the statutory U.S. federal income tax rate and the Company’s provision for income taxes are as follows:
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
Income tax provision at statutory federal and state tax rate |
|
|
21 | % |
|
|
21 | % |
|
Valuation allowance |
|
|
(21 | )% |
|
|
(21 | )% |
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
|
- |
|
|
|
- |
|
The net deferred income tax asset balance related to the following:
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
Net operating losses |
|
$ | 1,282,394 |
|
|
$ | - |
|
|
Deferred tax provision (credit) related to: |
|
|
|
|
|
|
|
|
|
Temporary differences |
|
|
|
|
|
|
|
|
|
Reward points |
|
|
9,983 |
|
|
|
- |
|
|
Adverse lease |
|
|
123,553 |
|
|
|
- |
|
|
Intangible assets |
|
|
58,260 |
|
|
|
- |
|
|
Stock Options |
|
|
78,287 |
|
|
|
- |
|
|
Allowance for doubtful accounts |
|
|
2,100 |
|
|
|
- |
|
|
Accrued compensation |
|
|
15,265 |
|
|
|
|
|
|
Loss carryforwards |
|
|
- |
|
|
|
- |
|
|
Valuation allowances |
|
|
(1,569,836 | ) |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
$ | - |
|
|
$ | - |
|
As of June 30, 2020, there were approximately $6,107,000 of losses available to reduce federal taxable income in future years and can be carried forward indefinitely.
Future realization of the tax benefits of existing temporary differences and net operating loss carryforwards ultimately depends on the existence of sufficient taxable income within the carryforward period. As of June 30, 2020, and 2019, the Company performed an evaluation to determine whether a valuation allowance was needed. The Company considered all available evidence, both positive and negative, which included the results of operations for the current and preceding years. The Company also considered whether there was any currently available information about future years. Because long-term contracts are not a significant part of the Company’s business, future results cannot be reliably predicted by considering past trends or by extrapolating past results. Moreover, the Company’s earnings are strongly influenced by national economic conditions and have been volatile in the past. Considering these factors, the Company determined that it was not possible to reasonably quantify future taxable income. The Company determined that it is more likely than not that all of the deferred tax assets will not be realized. Accordingly, the Company maintained a full valuation allowance as of June 30, 2020 and 2019.
| F-22 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
We file federal and state income tax returns in jurisdictions with varying statutes of limitations. Income tax returns generally remain subject to examination by federal and most state tax authorities. We are not currently under examination in any federal or state jurisdiction.
Note 14. Risks and Uncertainties
The Company does not have a concentration of revenues from any individual customer (more than 10%).
There is substantial uncertainty and different interpretations among federal, state and local regulatory agencies, legislators, academics and businesses as to the scope of operation of Farm Bill-compliant hemp programs relative to the emerging regulation of cannabinoids. These different opinions include, but are not limited to, the regulation of cannabinoids by the U.S. Drug Enforcement Administration, or DEA, and/or the FDA and the extent to which manufacturers of products containing Farm Bill-compliant cultivators and processors may engage in interstate commerce. The uncertainties cannot be resolved without further federal, and perhaps even state-level, legislation, regulation or a definitive judicial interpretation of existing legislation and rules. If these uncertainties continue, they may have an adverse effect upon the introduction of our products in different markets.
In December 2019, a novel strain of coronavirus (COVID-19) surfaced. The spread of COVID-19 around the world in the first quarter of 2020 has caused significant volatility in U.S. and international markets. There is significant uncertainty around the breadth and duration of business disruptions related to COVID-19, as well as its impact on the U.S. and international economies and, as such, the Company is unable to predict with certainty the potential impact of COVIT-19 on its business, results of operations, financial condition and cash flows.
Note 15. Segment Information
The Company provides the following distinctive services: (a) product sales and (b) trade show. These distinct services can be divided into two reportable segments, Product Sales and Trade Show Revenue. Selling, general and administrative expenses are not completely separately allocated among these two segments.
Unallocated Corporate expenses primarily include, certain executive compensation expenses and salaries, certain administrative salaries, corporate legal expenses, stock amortization expenses, consulting expenses, audit fees, corporate rent and facility costs, acquisition, integration and restructuring expenses and interest expense.
|
For the year ended June 30, 2020: |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 6,159,013 |
|
|
$ | 1,253,847 |
|
|
$ | - |
|
|
$ | 7,412,860 |
|
|
Costs |
|
|
4,280,909 |
|
|
|
561,988 |
|
|
|
- |
|
|
|
4,842,897 |
|
|
Gross profit |
|
|
1,878,104 |
|
|
|
691,859 |
|
|
|
- |
|
|
|
2,569,963 |
|
|
Gross margin |
|
|
30 | % |
|
|
55 | % |
|
|
|
|
|
|
35 | % |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
1,101,137 |
|
|
|
269,827 |
|
|
|
- |
|
|
370,964 |
| |
|
Impairment on leased equipment |
|
|
588,347 |
|
|
|
- |
|
|
|
- |
|
|
|
588,347 |
|
|
Gain on sale of equipment |
|
|
(180,211 | ) |
|
|
- |
|
|
|
- |
|
|
|
(180,211 | ) |
|
Depreciation and amortization |
|
|
611,346 |
|
|
|
- |
|
|
|
- |
|
|
|
611,346 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
175,225 |
|
|
|
4,486,426 |
|
|
|
4,661,651 |
|
|
Professional fees |
|
|
764,332 |
|
|
|
- |
|
|
|
- |
|
|
|
764,332 |
|
|
Interest expense, net |
|
|
- |
|
|
|
- |
|
|
|
138,406 |
|
|
|
138,406 |
|
|
Operating Expenses |
|
|
2,884,951 |
|
|
|
445,052 |
|
|
|
4,624,832 |
|
|
|
7,954,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | (799,181 | ) |
|
$ | 39,141 |
|
|
$ | (4,624,832 | ) |
|
$ | (5,384,872 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable - net |
|
|
165,147 |
|
|
|
- |
|
|
|
- |
|
|
|
165,147 |
|
|
Inventory |
|
|
1,448,448 |
|
|
|
- |
|
|
|
- |
|
|
|
1,448,448 |
|
|
Property and Equipment, net |
|
|
1,687,273 |
|
|
|
- |
|
|
|
- |
|
|
|
1,687,273 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,240,260 |
|
|
|
- |
|
|
|
- |
|
|
|
1,240,260 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
1,367,982 |
|
|
|
1,367,982 |
|
|
Total segment assets |
|
$ | 5,034,223 |
|
|
$ | - |
|
|
$ | 1,367,982 |
|
|
$ | 6,402,205 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 1,929,028 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,929,028 |
|
| F-23 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
For the year ended June 30, 2019: |
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 1,428,302 |
|
|
$ | 779,750 |
|
|
$ | - |
|
|
$ | 2,208,052 |
|
|
Costs |
|
|
945,756 |
|
|
|
226,099 |
|
|
|
- |
|
|
|
1,171,855 |
|
|
Gross profit |
|
|
482,546 |
|
|
|
553,651 |
|
|
|
- |
|
|
|
1,036,197 |
|
|
Gross margin |
|
|
34 | % |
|
|
71 | % |
|
|
|
|
|
|
47 | % |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
18,302 |
|
|
|
143,764 |
|
|
|
- |
|
|
|
162,066 |
|
|
Depreciation and amortization |
|
|
67,568 |
|
|
|
- |
|
|
|
- |
|
|
|
67,568 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
265,502 |
|
|
|
894,291 |
|
|
|
1,159,793 |
|
|
Professional fees |
|
|
209,815 |
|
|
|
26,008 |
|
|
|
- |
|
|
|
235,823 |
|
|
Other expense |
|
|
- |
|
|
|
- |
|
|
|
1,055 |
|
|
|
1,055 |
|
|
Interest income |
|
|
- |
|
|
|
- |
|
|
|
(3,068 | ) |
|
|
(3,068 | ) |
|
Operating Expenses |
|
|
295,686 |
|
|
|
435,273 |
|
|
|
892,278 |
|
|
|
1,623,237 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 186,860 |
|
|
$ | 118,378 |
|
|
$ | (892,278 | ) |
|
$ | (587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable - net |
|
|
127,722 |
|
|
|
- |
|
|
|
- |
|
|
|
127,722 |
|
|
Inventory |
|
|
1,138,064 |
|
|
|
- |
|
|
|
- |
|
|
|
1,138,064 |
|
|
Property and Equipment, net |
|
|
262,015 |
|
|
|
- |
|
|
|
- |
|
|
|
262,015 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,633,738 |
|
|
|
- |
|
|
|
- |
|
|
|
1,633,738 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
3,849,408 |
|
|
|
3,849,408 |
|
|
Total segment assets |
|
$ | 654,634 |
|
|
$ | - |
|
|
$ | 3,849,408 |
|
|
$ | 7,504,042 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 219,448 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 219,448 |
|
(a) The “other” segment is mainly related to the Company’s general expenses and overhead.
Note 16. Subsequent Events
No material events have occurred after June 30, 2020 that requires recognition or disclosure in the financial statements except as follows:
Acquisition of Infusionz LLC
On July 1, 2020 the Company entered into an Agreement and Plan of Merger with Infusionz LLC (the “Infusionz Agreement”) with the Members of Infusionz LLC (“Sellers”). Pursuant to the terms of the Infusionz Agreement on July 1, 2020 the Company acquired 100% of the outstanding interest of Infusionz LLC, a Colorado limited liability company (“Infusionz”).
Infusionz LLC (the “Company”) was formed in the state of Colorado in May 2016. The Company develops, manufactures and markets products based on Hemp-based Cannabidiol (“CBD”) including, but not limited to edibles, tinctures, topicals, capsules and pet products. The Company will also manufacture CBD products for other businesses under their brand and specifications.
Under the purchase method of accounting, the transaction was valued for accounting purposes at an estimated $3,350,000, which was the estimated fair value of the consideration paid by the Company. The estimate was based on the consideration paid or payable of $3,000,000 of common stock value and estimated cash of approximately $350,000, paid based on terms of the agreement. The Company will issue a minimum of 1,500,000 shares of common stock to the Sellers. At the close of the acquisition, the Company issued 400,000 shares of common stock, valued at the most recent issued price per common share of $0.85 per common share. Based on this valuation of the stock value, the Company will issue an additional 3,129,400 shares of common stock. The shares of common share will be adjusted based on the common share initial public offering price, as per the agreement. The Company issued 150,000 common shares and recorded an acquisition cost of $127,500 as a finder’s fee.
The assets and liabilities of Infusionz will be recorded at their respective fair values as of the closing date of the Infusionz Agreement, and the following table summarizes these values based on the estimated balance sheet at July 1, 2020, the effective closing date.
| F-24 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The intangibles will be recorded, based on the Company’s estimate of fair value, which are expected to consist primarily of customer lists with an estimated life of five to ten years and goodwill. Upon completion of a purchase price allocation and valuation, the allocation intangible assets will be adjusted accordingly.
|
Tangible Assets |
|
$ | 778,331 |
|
|
Intangible Assets |
|
|
1,855,873 |
|
|
Goodwill |
|
|
1,396,276 |
|
|
Liabilities Acquired |
|
|
(680,480 | ) |
|
Total Purchase Price |
|
$ | 3,350,000 |
|
Settlement of lease obligation
On December 1, 2020, the Company settled the lease obligation for the facility in Costa Mesa California and paid the settlement fee of $180,000 on December 4, 2020. The settlement resolved all the Company’s obligations for this facility. As of June 30, 2020, the Company had recorded $502,845 in operating lease payable and accrued lease payments of $64,721. Based on the June 30, 2020 accruals, the Company will realize a gain of approximately $387,556 from the settlement of the liability.
Other Subsequent Events
On November 1, 2020 the Company issued 182,500 shares of Common Stock in relations to the acquisition of Infusionz LLC. The shares were issued at a $0.85 per common share with adjustments to the final number of shares and value based on the acquisition agreement.
On December 7, 2020 the Company entered into a note agreement for total proceeds of $750,000 with a related party. The principal and interest of the note is payable in full in December 2022. The note bears interest at 2% and is unsecured. The Company expects to repay the note in full during February 2021.
On January 4, 2021 the Company paid the former members of Infusionz LLC $75,000 as per the acquisition agreement.
On February 1, 2021 the Company issued 182,500 shares of Common Stock in relations to the acquisition of Infusionz LLC. The shares were issued at a $0.85 per common share with adjustments to the final number of shares and value based on the acquisition agreement.
On February 2, 2021 the Company sold the 500,000 shares of Preferred Stock to Allan Marshall, CEO for net proceeds of $50,000.
On February 8, 2021, the Shareholders consented, and the Board of Directors approved the amendment of the Stock Option Plan to increase the maximum number of Shares that may be issued thereunder by 5,000,000 Shares to 10,000,000 Shares.
On February 8, 2021, the Shareholders consented, and the Board of Directors approved the Reverse Stock Split at the rate of 1 share of Common Stock for each 1.8 shares of Common Stock of the Company issued and outstanding (rounded up to the nearest whole number after giving effect to the Reverse Stock Split) on the Record Date of February 5, 2021.
On February 8, 2021, the Board of Directors approved the officers of the Company to file a Registration Statement on Form S-1 (the “Registration Statement”) to be prepared for the purposes of registering (i) up to $20,000,000 of Common Stock at a purchase price of no less than $4.50 per share (post reverse split), including an over-allotment option for the underwriter named therein (the “Underwriter”) to purchase additional shares of Common Stock amounting to 15% of the number of shares of Common Stock offered to the public; and (ii) a warrant to be issued to the Underwriter for the purchase of shares of Common Stock (the “Underwriter Warrant”); and (iii) the shares of Common Stock underlying the Underwriter Warrant (collectively, the “Securities”).
| F-25 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
GROVE |
|
UNAUDITED CONDENSED CONSOLDIATED BALANCE SHEETS (In U.S. Dollars, except share data or otherwise stated)
|
|
|
|
September 30, |
|
|
June 30, |
| ||
|
|
|
2020 |
|
|
2020 |
| ||
|
|
|
|
|
|
|
| ||
|
ASSETS |
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
| ||
|
Cash |
|
$ | 509,000 |
|
|
$ | 887,517 |
|
|
Accounts receivable, net (allowance for doubtful accounts was $25,000 and $10,000, respectively) |
|
|
474,312 |
|
|
|
165,147 |
|
|
Other receivables |
|
|
- |
|
|
|
72,000 |
|
|
Inventory |
|
|
1,893,111 |
|
|
|
1,448,448 |
|
|
Prepaid expenses |
|
|
115,283 |
|
|
|
76,562 |
|
|
Total current assets |
|
|
2,991,706 |
|
|
|
2,649,674 |
|
|
|
|
|
|
|
|
|
|
|
|
Property and Equipment, net |
|
|
1,649,194 |
|
|
|
1,687,273 |
|
|
Intangible asset, less accumulated amortization |
|
|
2,886,261 |
|
|
|
1,240,260 |
|
|
Goodwill |
|
|
1,889,371 |
|
|
|
493,095 |
|
|
Other assets |
|
|
37,068 |
|
|
|
37,068 |
|
|
Right-of-use asset |
|
|
327,848 |
|
|
|
294,835 |
|
|
Total other assets |
|
|
5,140,548 |
|
|
|
2,065,258 |
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ | 9,781,448 |
|
|
$ | 6,402,205 |
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ | 566,197 |
|
|
$ | 484,333 |
|
|
Accrued compensation |
|
|
260,727 |
|
|
|
195,399 |
|
|
Deferred revenue |
|
|
786,264 |
|
|
|
473,320 |
|
|
Accrued liabilities |
|
|
308,260 |
|
|
|
221,664 |
|
|
Acquisition payable |
|
|
2,810,000 |
|
|
|
- |
|
|
Current portion of notes payable |
|
|
250,612 |
|
|
|
183,595 |
|
|
Convertible note payable |
|
|
1,500,000 |
|
|
|
1,500,000 |
|
|
Current portion of operating lease payable |
|
|
540,657 |
|
|
|
461,123 |
|
|
Total current liabilities |
|
|
7,022,717 |
|
|
|
3,519,434 |
|
|
|
|
|
|
|
|
|
|
|
|
Notes payable, net of current portion |
|
|
595,433 |
|
|
|
365,350 |
|
|
Operating lease payable, net of current portion |
|
|
232,297 |
|
|
|
338,040 |
|
|
Total long-term liabilities |
|
|
827,730 |
|
|
|
703,390 |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 1,000,000 shares authorized, no shares issued and outstanding |
|
|
- |
|
|
|
- |
|
|
Common stock, $0.001 par value, 100,000,000 shares authorized, and 21,250,000 and 18,400,000 shares issued and outstanding, respectively |
|
|
21,250 |
|
|
|
18,400 |
|
|
Additional paid in capital |
|
|
9,817,808 |
|
|
|
7,306,164 |
|
|
Accumulated deficit |
|
|
(7,908,057 | ) |
|
|
(7,098,984 | ) |
|
Total stockholders' equity |
|
|
1,931,001 |
|
|
|
225,580 |
|
|
Non-controlling interest in subsidiary |
|
|
- |
|
|
|
1,953,801 |
|
|
Total equity |
|
|
1,931,001 |
|
|
|
2,179,381 |
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders' equity |
|
$ | 9,781,448 |
|
|
$ | 6,402,205 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-26 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
GROVE | |||
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (In U.S. Dollars, except share data or otherwise stated) |
|
|
|
Three Month's Ended September 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
|
|
|
|
|
|
| ||
|
Revenue |
|
|
2,937,442 |
|
|
|
1,669,163 |
|
|
|
|
|
2,937,442 |
|
|
|
1,669,163 |
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Revenue |
|
|
1,619,208 |
|
|
|
982,292 |
|
|
Gross profit |
|
|
1,318,234 |
|
|
|
686,871 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
365,258 |
|
|
|
304,880 |
|
|
General and administrative expenses |
|
|
1,583,641 |
|
|
|
1,237,958 |
|
|
Professional fees |
|
|
129,421 |
|
|
|
78,012 |
|
|
|
|
|
2,078,320 |
|
|
|
1,620,850 |
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(760,086 | ) |
|
|
(933,979 | ) |
|
|
|
|
|
|
|
|
|
|
|
Other expense (income), net |
|
|
|
|
|
|
|
|
|
Other expense (income), net |
|
|
6,296 |
|
|
|
- |
|
|
Interest expense (income), net |
|
|
42,691 |
|
|
|
(625 | ) |
|
Other expense (income), net |
|
|
48,987 |
|
|
|
(625 | ) |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
(809,073 | ) |
|
|
(933,354 | ) |
|
|
|
|
|
|
|
|
|
|
|
Earnings per share attributable to Grove, Inc. common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
$ | (0.04 | ) |
|
$ | (0.05 | ) |
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding |
|
|
18,691,991 |
|
|
|
17,563,619 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-27 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
GROVE | ||||||||||||||||
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (In U.S. Dollars, except share data or otherwise stated)
|
|
|
|
Preferred |
|
|
Preferred |
|
|
Common |
|
|
Common |
|
|
Additional |
|
|
|
|
Non- |
|
|
Total |
| |||||||||
|
|
|
Stock |
|
|
Stock |
|
|
Stock |
|
|
Stock |
|
|
Paid |
|
|
Accumulated |
|
|
controlling |
|
|
Shareholders' |
| ||||||||
|
|
|
Shares |
|
|
Par |
|
|
Shares |
|
|
Par |
|
|
In Capital |
|
|
Deficit |
|
|
Interest |
|
|
Equity |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Balance, June 30, 2019 |
|
|
500,000 |
|
|
$ | 500 |
|
|
|
17,376,470 |
|
|
$ | 17,376 |
|
|
$ | 6,438,918 |
|
|
$ | (1,715,311 | ) |
|
$ | 1,655,000 |
|
|
$ | 6,396,483 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock for cash |
|
|
(500,000 | ) |
|
|
(500 | ) |
|
|
500,000 |
|
|
|
500 |
|
|
|
50,000 |
|
|
|
- |
|
|
|
- |
|
|
|
50,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for cash |
|
|
- |
|
|
|
- |
|
|
|
23,530 |
|
|
|
24 |
|
|
|
19,976 |
|
|
|
- |
|
|
|
|
|
|
|
20,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trunano Labs, Inc. subsidiary stock issued for cash |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
300,000 |
|
|
|
300,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
93,193 |
|
|
|
- |
|
|
|
- |
|
|
|
93,193 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(587,040 | ) |
|
|
- |
|
|
|
(587,040 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2019 |
|
|
- |
|
|
$ | - |
|
|
|
17,900,000 |
|
|
$ | 17,900 |
|
|
$ | 6,602,087 |
|
|
$ | (2,302,351 | ) |
|
$ | 1,955,000 |
|
|
$ | 6,272,636 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2020 |
|
|
- |
|
|
$ | - |
|
|
|
18,400,000 |
|
|
$ | 18,400 |
|
|
$ | 7,306,164 |
|
|
$ | (7,098,984 | ) |
|
$ | 1,953,801 |
|
|
$ | 2,179,381 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of Trunano Labs, Inc. subsidiary stock into Grove common stock |
|
|
- |
|
|
|
- |
|
|
|
2,300,000 |
|
|
|
2,300 |
|
|
|
1,951,501 |
|
|
|
- |
|
|
|
(1,953,801 | ) |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for acquisition |
|
|
- |
|
|
|
- |
|
|
|
400,000 |
|
|
|
400 |
|
|
|
339,600 |
|
|
|
- |
|
|
|
- |
|
|
|
340,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for acquisition costs |
|
|
|
|
|
|
|
|
|
|
150,000 |
|
|
|
150 |
|
|
|
127,350 |
|
|
|
- |
|
|
|
- |
|
|
|
127,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
93,193 |
|
|
|
- |
|
|
|
- |
|
|
|
93,193 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(809,073 | ) |
|
|
- |
|
|
|
(809,073 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2020 |
|
|
- |
|
|
$ | - |
|
|
|
21,250,000 |
|
|
$ | 21,250 |
|
|
$ | 9,817,808 |
|
|
$ | (7,908,057 | ) |
|
$ | - |
|
|
$ | 1,931,001 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-28 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOW (In U.S. Dollars, except share data or otherwise stated) |
|
|
|
Three months ended September 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Cash flows from operating activities |
|
|
|
|
|
| ||
|
Net loss |
|
$ | (809,073 | ) |
|
$ | (933,354 | ) |
|
Adjustments to reconcile net loss to net cash used by operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
279,899 |
|
|
|
114,037 |
|
|
Issuance of common stock for acquisition costs |
|
|
127,500 |
|
|
|
- |
|
|
Provision for doubtful accounts and bad debt expense |
|
|
1,165 |
|
|
|
34,707 |
|
|
Loss on sale of equipment |
|
|
6,292 |
|
|
|
- |
|
|
Stock based compensation |
|
|
93,193 |
|
|
|
93,193 |
|
|
Changes in assets and liabilities, net of acquired amounts |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(257,858 | ) |
|
|
66,537 |
|
|
Other receivables |
|
|
- |
|
|
|
27,944 |
|
|
Inventory |
|
|
(269,818 | ) |
|
|
(843,745 | ) |
|
Prepaid expenses and other assets |
|
|
(30,231 | ) |
|
|
(920,168 | ) |
|
Accounts payable and accrued liabilities |
|
|
21,225 |
|
|
|
347,604 |
|
|
Deferred revenue |
|
|
200,521 |
|
|
|
- |
|
|
Net cash used in operating activities |
|
|
(637,185 | ) |
|
|
(2,013,245 | ) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Acquisition of Infusionz LLC., net of cash acquired |
|
|
212,122 |
|
|
|
- |
|
|
Proceeds from sale of property and equipment |
|
|
64,000 |
|
|
|
- |
|
|
Acquisition of property and equipment |
|
|
(5,454 | ) |
|
|
(1,043,472 | ) |
|
Net cash provided by (used in) investing activities |
|
|
270,668 |
|
|
|
(1,043,472 | ) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net |
|
|
- |
|
|
|
70,000 |
|
|
Proceeds from issuance of non-controlling interest |
|
|
- |
|
|
|
300,000 |
|
|
Repayment of notes payable |
|
|
(12,000 | ) |
|
|
- |
|
|
Net cash (used in) provided by financing activities |
|
|
(12,000 | ) |
|
|
370,000 |
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease in cash |
|
|
(378,517 | ) |
|
|
(2,686,717 | ) |
|
Cash, beginning of period |
|
|
887,517 |
|
|
|
3,697,432 |
|
|
Cash, end of period |
|
$ | 509,000 |
|
|
$ | 1,010,715 |
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental cash flow disclosures |
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ | - |
|
|
$ | - |
|
|
Income tax paid |
|
$ | - |
|
|
$ | - |
|
|
Non-cash financing activities |
|
|
|
|
|
|
|
|
|
Issuance of stock for the acquisition of Infusionz, LLC |
|
$ | 340,000 |
|
|
$ | - |
|
|
Repayment of Infusionz LLC debt to Grove, Inc. |
|
$ | 72,000 |
|
|
$ | - |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-29 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Notes to Unaudited Condensed Consolidated Financial Statements
Three Months Ended September 30, 2020 and 2019
Note 1. Background Information
We are in the business of developing, producing, marketing, and selling raw materials, white label products and end consumer products containing the hemp plant extract, Cannabidiol (“CBD”). We sell to numerous consumer markets including the nutraceutical, beauty care, pet care and functional food sectors. We seek to take advantage of an emerging worldwide trend to re-energize the production of industrial hemp and to foster its many uses for consumers.
In addition, we are an operator of an annual tradeshow in the United States related to the CBD industry. The trade show business is naturally seasonal. The Company only has one trade show, CBD.IO, which is held in November each year. Because event revenue is recognized when a particular event is held, the Company experiences fluctuations in quarterly revenue based on the completion of the trade show event. The seasonality of the business is typical of the trade show industry.
Grove Inc. (the “Company”) is a Nevada corporation and has seven wholly owned subsidiaries, Trunano Labs, Inc., a Nevada corporation, Cresco Management LLC, a California limited liability company, Steam Distribution, LLC, a California limited liability company; One Hit Wonder, Inc., a California corporation; Havz, LLC, d/b/a Steam Wholesale; a California limited liability company, One Hit Wonder Holdings, LLC a California limited liability company and SWCH LLC; a Delaware limited liability company.
On July 1, 2020, the noncontrolling shareholders of the Company’s subsidiary, Trunano Labs, Inc., converted 1,761,261 shares of Trunano Labs, Inc. stock, representing all the outstanding stock by minority interest holders, into 2,300,000 shares of Grove Inc. common stock. As of July 1, 2020, Trunano Labs, Inc. is a wholly owned subsidiary of Grove Inc.
On July 1, 2020, the Company entered into an Agreement and Plan of Merger with Infusionz LLC (the “Infusionz Agreement”) with the members of Infusionz LLC (the “Sellers”). Pursuant to the terms of the Infusionz Agreement, on July 1, 2020, the Company acquired 100% of the outstanding membership interests of Infusionz LLC, a Colorado limited liability company (“Infusionz”).
Liquidity and Going Concern
The Company experienced significant net losses in the year’s ended June 30, 2020 and 2019. Management has implemented a strategy which includes cost reductions and consolidation of certain operating activities to gain efficiencies as well as identifying strategic acquisitions, financed primarily through a combination of the issuance of equity and debt, to improve the overall profitability and cash flows of the Company. As of April 1, 2020, the Company ceased production operations in California and has consolidated operations into a single location in Nevada. These factors raise substantial doubts about the Company’s ability to continue as a going concern. The condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event that the Company cannot continue as a going concern.
As of September 30, 2020, the Company had cash of approximately $509,000 and a working capital deficit of approximately $4,031,011.
The ability to continue as a going concern is dependent upon the Company generating profitable operations in the future and/or obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. Management intends to finance operating costs over the next twelve months from the date of the issuance of these condensed consolidated financial statements with existing cash on hand and/or the private placement of common stock. There is, however, no assurance that the Company will be able to raise any additional capital through any type of offering on terms acceptable to the Company, as existing cash on hand will be insufficient to finance operations over the next twelve months.
| F-30 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Basis of Presentation and Principles of Consolidation
The Company’s condensed consolidated financial statements are prepared in accordance with accounting principles general accepted in the United States (“GAAP”). The condensed consolidated financial statements include the accounts of all subsidiaries in which the Company holds a controlling financial interest as of September 30, 2020 and June 30, 2020.
In the opinion of management, the unaudited interim condensed consolidated financial statements reflect all adjustments of a normal recurring nature that are necessary for a fair presentation of the results for the interim periods presented. All significant intercompany transactions and balances are eliminated in consolidation. However, the results of operations included in such financial statements may not necessary be indicative of annual results.
The Company uses the same accounting policies in preparing quarterly and annual financial statements. Certain information and footnote disclosures normally included in the annual consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) have been condensed or omitted. These unaudited condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto included in the Company’s annual audited consolidated financial statements as of and for the years ended June 30, 2020 and 2019 incorporated in this registration statement.
Note 2. Acquisitions
Infusionz LLC
On July 1, 2020 the Company entered into an Agreement and Plan of Merger with Infusionz LLC (the “Infusionz Agreement”) with the Members of Infusionz LLC (“Sellers”). Pursuant to the terms of the Infusionz Agreement on July 1, 2020 the Company acquired 100% of the outstanding interest of Infusionz LLC, a Colorado limited liability company (“Infusionz”).
Infusionz was formed in the state of Colorado in May 2016. The Infusionz develops, manufactures, and markets products based on Hemp-based Cannabidiol (“CBD”) including, but not limited to edibles, tinctures, topicals, capsules and pet products. Infusionz will also manufacture CBD products for other businesses under their brand and specifications.
Under the purchase method of accounting, the transaction was valued for accounting purposes at an estimated $3,350,000, which was the estimated fair value of the consideration paid by the Company. The estimate was based on the consideration paid or payable of $3,000,000 of common stock value and estimated cash of approximately $350,000, paid based on terms of the agreement. The Company will issue a minimum of 1,500,000 shares of common stock to the Sellers. On July 1, 2020, the close of the acquisition, the Company issued 400,000 shares of common stock, valued at the most recent issued price per common share of $0.85 per common stock. Based on this valuation of the stock value, the Company will issue an additional 3,129,400 shares of common stock. The shares of common share will be adjusted based on the common share initial public offering price, as per the agreement. The Company issued 150,000 common shares and recorded an acquisition cost of $127,500 as a finder’s fee.
The intangibles will be recorded, based on the Company’s estimate of fair value, which are expected to consist primarily of customer lists with an estimated life of five to ten years and goodwill. Upon completion of a purchase price allocation, the allocation intangible assets will be adjusted accordingly.
The assets and liabilities of Infusionz will be recorded at their respective fair values as of the closing date of the Infusionz Agreement, and the following table summarizes these values based on the estimated balance sheet on July 1, 2020, the effective closing date.
|
Assets Purchased |
|
$ | 778,331 |
|
|
Liabilities Assumed |
|
|
(680,480 | ) |
|
Net Assets Purchased |
|
|
97,851 |
|
|
Purchase Price |
|
|
(3,350,000 | ) |
|
Intangible Assets and Goodwill from Purchase |
|
$ | 3,402,149 |
|
|
Customer Relationships |
|
$ | 1,477,123 |
|
|
Trade Name |
|
|
378,750 |
|
|
Goodwill |
|
|
1,396,276 |
|
|
Intangible Assets and Goodwill from Purchase |
|
$ | 3,402,149 |
|
| F-31 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Note 3. Inventory
Inventory consisted of the following:
|
|
|
September 30, 2020 |
|
|
June 30, 2020 |
| ||
|
Raw materials |
|
$ | 1,146,236 |
|
|
$ | 730,000 |
|
|
Finished goods |
|
|
746,875 |
|
|
|
718,448 |
|
|
|
|
$ | 1,893,111 |
|
|
$ | 1,448,448 |
|
The Company writes-off the value of inventory deemed excessive or obsolete. As of September 30, 2020, and June 30, 2020, the Company did not deem that an inventory write-off was considered necessary.
Note 4. Property and Equipment
Property and equipment consist of the following:
|
|
|
September 30, 2020 |
|
|
June 30, 2020 |
| ||
|
Furniture and fixtures |
|
$ | 8,667 |
|
|
$ | 4,167 |
|
|
Computer equipment |
|
|
51,488 |
|
|
|
48,606 |
|
|
Manufacturing equipment |
|
|
46,997 |
|
|
|
45,692 |
|
|
Leasehold improvements |
|
|
1,815,064 |
|
|
|
1,787,200 |
|
|
Vehicles |
|
|
9,500 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, gross |
|
|
1,931,716 |
|
|
|
1,885,665 |
|
|
Less accumulated depreciation |
|
|
(282,522 | ) |
|
|
(198,392 | ) |
|
Property and equipment, net |
|
$ | 1,649,194 |
|
|
$ | 1,687,273 |
|
During the three months ended September 30, 2020, the Company sold manufacturing equipment with a carrying value of approximately $79,999 for cash proceeds of $64,000 which resulting in a loss on the disposal of approximately $6,292.
Depreciation expense for the three months ended September 30, 2020 and 2019 was $70,027 and $15,072, respectively.
Note 5. Intangible Assets
As of September 30, 2020
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Loss on Impairment of Intangible Assets |
|
|
Net Book Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Customer relationships |
|
$ | 2,676,383 |
|
|
$ | 492,072 |
|
|
$ | - |
|
|
$ | 2,184,311 |
|
|
Trade name |
|
|
845,305 |
|
|
|
143,355 |
|
|
|
- |
|
|
|
701,950 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ | 3,521,688 |
|
|
$ | 635,427 |
|
|
$ | - |
|
|
$ | 2,886,261 |
|
| F-32 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
As of June 30, 2020
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Loss on Impairment of Intangible Assets |
|
|
Net Book Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Customer relationships |
|
$ | 1,199,260 |
|
|
$ | 324,467 |
|
|
$ | - |
|
|
$ | 874,793 |
|
|
Trade name |
|
|
466,555 |
|
|
|
101,088 |
|
|
|
- |
|
|
|
365,467 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ | 1,665,815 |
|
|
$ | 425,555 |
|
|
$ | - |
|
|
$ | 1,240,260 |
|
For the three months ended September 30, 2020 and 2019, the Company amortized approximately $209,872 and $393,126, respectively, related to the customer list and trade name intangible asset. The customer list is being amortized on a straight-line basis over 4 years. The trade names are being amortized on a straight-line basis over 5 years.
|
Future amortization of intangible assets are as follows: |
|
|
| |
|
|
|
|
| |
|
June 30, 2021 |
|
$ | 620,066 |
|
|
June 30, 2022 |
|
|
829,939 |
|
|
June 30, 2023 |
|
|
829,939 |
|
|
June 30, 2024 |
|
|
530,567 |
|
|
June 30, 2025 |
|
|
75,750 |
|
|
|
|
$ | 2,886,261 |
|
Note 6. Operating Leases
During November 2019, the Company entered into a lease for a Nevada facility that commenced on November 13, 2019 and recorded a right of use asset and corresponding lease liability. Lease expense was $37,995 for the three months ended September 30, 2020.
During July 2019, the Company entered a lease for a California facility that commenced on July 1, 2019 and recorded a right of use asset and corresponding lease liability. In March 2020, the Company consolidated operations to its Nevada facility and abandoned its manufacturing and sales facility in Costa Mesa. For the year ended June 30, 2020 the Company recorded an impairment loss of $558,918 and subsequently negotiated a settlement for this liability and recognized a gain of $387,860 in December of 2020.
The Company has one leased facility the Company is presentation using, which is office, manufacturing, and warehouse space. The Company is responsible for real estate taxes, utilities, and repairs under the terms of certain of the operating leases. Therefore, all lease and non-lease components are combined and accounted for as single lease component.
During November 2018, the Company entered into a new lease for equipment that commenced on November 1, 2018 and recorded a right of use asset and corresponding lease liability. Lease expense was $7,866 for the three months ended September 30, 2020.
For month to month leases and leases that expire in one year or less, lease expense for the three months ended September 30, 2020 was $49,185.
| F-33 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The Company’s weighted average remaining lease term and weighted average discount rate for operating leases as of September 30, 2020 are:
|
Weighted average remaining lease term |
|
14 Months |
| |
|
Weighted average incremental borrowing rate |
|
|
5.0 | % |
For the three months ended September 30, 2020, the components of lease expense, included in general and administrative expenses and interest expense in the condensed consolidated statements of operations income, are as follows:
Operating lease cost:
|
Operating lease cost |
|
$ | 130,284 |
|
|
Amortization of ROU assets |
|
$ | 120,936 |
|
|
Interest expense |
|
|
9,347 |
|
|
Total lease cost |
|
$ | 260,567 |
|
The table below reconciles the undiscounted future minimum lease payments (displayed by year and in the aggregate) under noncancelable operating leases with terms of more than one year to the total operating lease liabilities recognized in the condensed consolidated balance sheet as of September 30, 2020:
|
2021 |
|
|
565,198 |
|
|
2022 |
|
|
226,868 |
|
|
2023 |
|
|
6,744 |
|
|
2024 |
|
|
2,810 |
|
|
Total undiscounted future minimum lease payments |
|
|
801,620 |
|
|
Less: Imputed interest |
|
|
(27,538 | ) |
|
Present value of operating lease obligation |
|
|
774,082 |
|
Note 7. Accrued Liabilities
Accrued liabilities consist of the following:
|
|
|
September 30, 2020 |
|
|
June 30, 2020 |
| ||
|
Accrued expenses for loyalty program |
|
$ | 47,907 |
|
|
$ | 47,400 |
|
|
Accrued rent |
|
|
64,721 |
|
|
|
- |
|
|
Accrued interest |
|
|
128,286 |
|
|
|
93,543 |
|
|
Other accrued liabilities |
|
|
67,346 |
|
|
|
80,721 |
|
|
|
|
$ | 308,260 |
|
|
$ | 221,664 |
|
Note 8. Convertible Promissory Notes and Notes Payable
During October of 2019, the Company entered into convertible promissory notes (Notes) for total proceeds of $1,500,000. The principal and interest of the Notes are payable in full at the maturity date of April 2021, if not previously converted. The Notes have an interest rate of 8%, total accrued interest is to be repaid at maturity, and are convertible into common stock if the Company enters a “Financing” arrangement which results in the Company’s common stock becoming listed or trading. The conversion rate would be equal to the price of the Company’s common stock sold in the “Financing”.
On April 28, 2020, the Company entered a Paycheck Protection Program loan for $398,945 in connection with COVID-19. The promissory note has a fixed payment schedule, commencing seven months following the funding of the note and consisting of seventeen monthly payments of principal and interest, with the principal component of each payment based upon the level of amortization of principal over a two year period from the funding date. A final payment for the unpaid principal and accrued interest will be payable no later than April 28, 2022. The note bears interest at a rate of 1.00% per annum and is deferred for the first six months of the loan. Certain portions of the loan may qualify for loan forgiveness based on the terms of the program.
| F-34 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
On May 13, 2020, Infusionz entered a Paycheck Protection Program loan for $297,100 in connection with COVID-19. The promissory note has a fixed payment schedule, commencing seven months following the funding of the note and consisting of seventeen monthly payments of principal and interest, with the principal component of each payment based upon the level of amortization of principal over a two year period from the funding date. A final payment for the unpaid principal and accrued interest will be payable no later than May 13, 2022. The note bears interest at a rate of 1.00% per annum and is deferred for the first six months of the loan. Certain portions of the loan may qualify for loan forgiveness based on the terms of the program.
On June 3, 2020, the Company entered a loan for $150,000 with the Small Business Administration. The promissory note as a fixed payment schedule commencing on June 3, 2021, consisting of principal and interest payments of $731 monthly. The balance of the principal and interest will payable thirty years from the date of the promissory note. The note bears interest at a rate of 3.75% per annum. The loan is collateralized by any and all tangible and intangible properties of the Company.
Convertible promissory notes and notes payable outstanding as of September 30, 2020 are summarized below:
|
|
|
Maturity Date |
|
September 30, 2020 |
| |
|
8% $1,500,000 Convertible Promissory Notes |
|
April 2021 |
|
$ | 1,500,000 |
|
|
3.75% $150,000 Note Payable |
|
June 2050 |
|
|
150,000 |
|
|
1% $398,945 Note Payable |
|
April 2022 |
|
|
398,945 |
|
|
1% $297,100 Note Payable |
|
May 2022 |
|
|
297,100 |
|
|
Total notes payable |
|
|
|
|
2,346,045 |
|
|
Less current portion of notes payable |
|
|
|
|
1,750,612 |
|
|
Notes payable, less current portion |
|
|
|
$ | 595,433 |
|
|
Future payments on convertible promissory notes and notes payable are as follows: |
|
|
| |
|
|
|
|
| |
|
June 30, 2021 |
|
$ | 1,768,224 |
|
|
June 30, 2022 |
|
|
371,984 |
|
|
June 30, 2023 |
|
|
74,112 |
|
|
June 30, 2024 |
|
|
8,772 |
|
|
June 30, 2025 |
|
|
8,772 |
|
|
Thereafter |
|
|
114,181 |
|
|
|
|
$ | 2,346,045 |
|
Note 9. Related Party Transactions
For the three months ended September 30, 2019, the Company leased the Las Vegas warehouse from a shareholder for $22,071 per month. This lease ended December 31, 2019 and there were no further liabilities related to this lease. The owner of the warehouse is also related to one of the members of management.
During the three months ended September 30, 2020 the Company contracted with J Charles Management to provide sales services of approximately $3,481. J Charles Management is partially owned by a member of Infusionz LLC.
During the three months ended September 30, 2020 the Company paid expenses to NRW Ventures LLC of approximately $9,420 as reimbursement for operational expenses paid by Reido Distributors on behalf of the Company. NRW Ventures LLC is partially owned by the current CEO and owner of Infusionz LLC.
During the three months ended September 30, 2020 the Company paid expenses to Reido Distributors of approximately $23,686 as reimbursement for operational expenses paid by Reido Distributors on behalf of the Company. Reido Distributors is partially owned by a member of Infusionz LLC.
| F-35 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
During the three months ended September 30, 2020 the Company repaid a note from one of the members of management. The loan was $12,000 and was due upon demand.
The above related party transactions are not necessarily indicative of the amounts and terms that would have been incurred had comparable transactions been entered into with independent parties.
Note 10. Equity Transactions
Preferred Stock
The Company’s Board of Directors has authorized 1,000,000 shares of preferred stock with a par value of $0.001 and issued 500,000 shares of preferred stock. This preferred stock is convertible into a single share of common stock at a price of $0.05 per share of preferred stock with additional terms and conditions determined by the Board of Directors. During the year ended June 30, 2020, an investor converted 500,000 shares of preferred stock into 500,000 shares of common stock for cash proceeds of $50,000.
Common Stock
During the three months ended September 30, 2019, the Company issued 23,530 shares of common stock for cash of $20,000.
During the three months ended September 30, 2020, the Company issued 400,000 shares of common stock for the acquisition of Infusionz. The shares were valued at $340,000 or $0.85 per share, as this was the last transaction price. In addition, the Company issued 150,000 shares of common stock valued at $127,500 for acquisition costs.
Trunano Labs, Inc. Common Stock
Trunano Labs, Inc. has 10,000,000 shares of common stock authorized with a par value of $0.001. As of June 30, 2020, Trunano Labs, Inc, had 7,261,261 issued and outstanding shares of common stock, of which 5,770,270 is owned by the Company. During the three months ended September 30, 2019, Trunano Labs, Inc. issued 270,270 shares of common stock for cash proceeds of approximately $300,000. Primarily due to the decline in CBD isolate price, there were no operations during the three months ended September 30, 2020 or 2019 for Trunano Labs, Inc.
On July 1, 2020 the Company and Trunano Labs, Inc. converted 1,761,261, representing all of the outstanding stock by minority interest holders, to 2,300,000 of Grove Inc. common stock. As of July 1, 2020, Trunano Labs, Inc. is a wholly owned subsidiary of Grove Inc.
Note 11. Stock Based Compensation
The Board of Directors of the Company may from time to time, in its discretion grant to directors, officers, consultants and employees of the Company, non-transferable options to purchase common shares. The options are exercisable for a period of up to 10 years from the date of the grant.
The following table reflects the continuity of stock options for the three months ended September 30, 2020:
A summary of stock option activity is as follows:
|
|
|
|
|
Weighted |
|
|
Average |
|
|
| ||||||
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Aggregated |
| |||||
|
|
|
Options |
|
|
Exercise |
|
|
Contractual |
|
|
Intrinsic |
| ||||
|
|
|
Outstanding |
|
|
Price |
|
|
Life (Years) |
|
|
Value |
| ||||
|
Outstanding at June 30, 2020 |
|
|
1,800,000 |
|
|
$ | 0.85 |
|
|
|
8.5 |
|
|
$ | - |
|
|
Granted |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Expired |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Outstanding at September 30, 2020 |
|
|
1,800,000 |
|
|
$ | 0.85 |
|
|
|
8.5 |
|
|
|
- |
|
|
Options exercisable at September 30, 2020 (vested) |
|
|
1,366,667 |
|
|
|
0.85 |
|
|
|
8.5 |
|
|
|
- |
|
| F-36 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Stock-based compensation expense attributable to stock options was approximately $93,193 and $93,193 for the three months ended September 30, 2020 and 2019, respectively. As of September 30, 2020, there was approximately $248,514 of unrecognized compensation expense related to unvested stock options outstanding, and the weighted average vesting period for those options was 1 years.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on historical experience of forfeitures, the Company estimated forfeitures at 0% for each of the three months ended September 30, 2020 and 2019, respectively.
Note 12. Risks and Uncertainties
The Company does not have a concentration of revenues from any individual customer (more than 10%).
There is substantial uncertainty and different interpretations among federal, state and local regulatory agencies, legislators, academics and businesses as to the scope of operation of Farm Bill-compliant hemp programs relative to the emerging regulation of cannabinoids. These different opinions include, but are not limited to, the regulation of cannabinoids by the U.S. Drug Enforcement Administration, or DEA, and/or the FDA and the extent to which manufacturers of products containing Farm Bill-compliant cultivators and processors may engage in interstate commerce. The uncertainties cannot be resolved without further federal, and perhaps even state-level, legislation, regulation or a definitive judicial interpretation of existing legislation and rules. If these uncertainties continue, they may have an adverse effect upon the introduction of our products in different markets.
In December 2019, a novel strain of coronavirus (COVID-19) surfaced. The spread of COVID-19 around the world in 2020 has caused significant volatility in U.S. and international markets. There is significant uncertainty around the breadth and duration of business disruptions related to COVID-19, as well as its impact on the U.S. and international economies and, as such, the Company is unable to predict with certainty the potential impact of COVIT-19 on its business, results of operations, financial condition, and cash flows.
Note 13. Segment Information
The Company provides the following distinctive services: (a) product sales and (b) trade show. These distinct services can be divided into two reportable segments, Product Sales and Trade Show Revenue. Selling, general and administrative expenses are not completely separately allocated among these two segments.
Unallocated Corporate expenses primarily include, certain executive compensation expenses and salaries, certain administrative salaries, corporate legal expenses, stock amortization expenses, consulting expenses, audit fees, corporate rent and facility costs, acquisition, integration and restructuring expenses and interest expense.
For the three month’s ended September 30, 2020:
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 2,937,442 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 2,937,442 |
|
|
Costs |
|
|
1,619,208 |
|
|
|
- |
|
|
|
- |
|
|
|
1,619,208 |
|
|
Gross profit |
|
|
1,318,234 |
|
|
|
- |
|
|
|
- |
|
|
|
1,318,234 |
|
|
Gross margin |
|
|
45 | % |
|
|
0 | % |
|
|
0 | % |
|
|
45 | % |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
365,258 |
|
|
|
- |
|
|
|
- |
|
|
|
365,258 |
|
|
Loss on sale of equipment |
|
|
6,292 |
|
|
|
- |
|
|
|
- |
|
|
|
6,292 |
|
|
Depreciation and amortization |
|
|
279,899 |
|
|
|
- |
|
|
|
- |
|
|
|
279,899 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
- |
|
|
|
1,303,742 |
|
|
|
1,303,742 |
|
|
Professional fees |
|
|
129,421 |
|
|
|
- |
|
|
|
- |
|
|
|
129,421 |
|
|
Other expense |
|
|
- |
|
|
|
- |
|
|
|
4 |
|
|
|
4 |
|
|
Interest expense, net |
|
|
42,691 |
|
|
|
- |
|
|
|
- |
|
|
|
42,691 |
|
|
Operating Expenses |
|
|
823,561 |
|
|
|
- |
|
|
|
1,303,746 |
|
|
|
2,127,307 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 494,669 |
|
|
$ | - |
|
|
$ | (1,303,742 | ) |
|
$ | (809,073 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
474,312 |
|
|
|
- |
|
|
|
- |
|
|
|
474,312 |
|
|
Inventory |
|
|
1,893,111 |
|
|
|
- |
|
|
|
- |
|
|
|
1,893,111 |
|
|
Property and Equipment, net |
|
|
1,649,194 |
|
|
|
- |
|
|
|
- |
|
|
|
1,649,194 |
|
|
Intangible asset, less accumulated amortization |
|
|
2,886,261 |
|
|
|
- |
|
|
|
- |
|
|
|
2,886,261 |
|
|
Goodwill |
|
|
1,889,371 |
|
|
|
- |
|
|
|
- |
|
|
|
1,889,371 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
9,199 |
|
|
|
989,199 |
|
|
Total segment assets |
|
$ | 8,792,249 |
|
|
$ | - |
|
|
$ | 989,199 |
|
|
$ | 9,781,448 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 5,454 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 5,454 |
|
| F-37 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
For the three month’s ended September 30, 2019:
|
|
|
Product |
|
|
Trade Show |
|
|
Other (a) |
|
|
Total |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ | 1,669,163 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,669,163 |
|
|
Costs |
|
|
982,292 |
|
|
|
- |
|
|
|
- |
|
|
|
982,292 |
|
|
Gross profit |
|
|
686,871 |
|
|
|
0 |
|
|
|
- |
|
|
|
686,871 |
|
|
Gross margin |
|
|
41 | % |
|
|
0 | % |
|
|
0 |
|
|
%41% |
| |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
304,880 |
|
|
|
- |
|
|
|
- |
|
|
|
304,880 |
|
|
Depreciation and amortization |
|
|
114,037 |
|
|
|
- |
|
|
|
- |
|
|
|
114,037 |
|
|
General and administrative expenses |
|
|
- |
|
|
|
- |
|
|
|
1,123,921 |
|
|
|
1,123,921 |
|
|
Professional fees |
|
|
78,012 |
|
|
|
- |
|
|
|
- |
|
|
|
78,012 |
|
|
Interest income |
|
|
- |
|
|
|
- |
|
|
|
(625 |
|
|
)(625) |
| |
|
Operating Expenses |
|
|
496,929 |
|
|
|
- |
|
|
|
1,123,296 |
|
|
|
1,620,225 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ | 189,942 |
|
|
$ | - |
|
|
$ | (1,123,296 |
|
|
)$(933,354) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable – net |
|
|
26,478 |
|
|
|
- |
|
|
|
- |
|
|
|
26,478 |
|
|
Inventory |
|
|
1,981,809 |
|
|
|
- |
|
|
|
- |
|
|
|
1,981,809 |
|
|
Property and Equipment, net |
|
|
1,290,415 |
|
|
|
- |
|
|
|
- |
|
|
|
1,290,415 |
|
|
Intangible asset, less accumulated amortization |
|
|
1,534,773 |
|
|
|
- |
|
|
|
- |
|
|
|
1,534,773 |
|
|
Goodwill |
|
|
493,095 |
|
|
|
- |
|
|
|
- |
|
|
|
493,095 |
|
|
Unallocated assets |
|
|
- |
|
|
|
- |
|
|
|
2,085,930 |
|
|
|
2,085,930 |
|
|
Total segment assets |
|
$ | 5,326,570 |
|
|
$ | - |
|
|
$ | 2,085,930 |
|
|
$ | 7,412,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenditures for segment assets |
|
$ | 1,043,472 |
|
|
$ | - |
|
|
$ | - |
|
|
$ | 1,043,472 |
|
(a) The “other” segment is mainly related to the Company’s general expenses and overhead.
Note 14. Subsequent Events
On December 1, 2020, the Company settled the lease obligation for the facility in Costa Mesa California and paid the settlement fee of $180,000 on December 4, 2020. The settlement resolved all the Company’s obligations for this facility. As of June 30, 2020, the Company had recorded $502,845 in operating lease payable and accrued lease payments of $64,721. On December 1, 2020, the Company realized a gain of approximately $387,860 from the settlement of the liability.
On November 1, 2020 the Company issued 182,500 shares of Common Stock in relations to the acquisition of Infusionz LLC. The shares were issued at a $0.85 per common share with adjustments to the final number of shares and value based on the acquisition agreement.
On December 7, 2020 the Company entered into a note agreement for total proceeds of $750,000 with a related party. The principal and interest of the note is payable in full in December 2022. The note bears interest at 2% and is unsecured. The Company expects to repay the note in full during February 2021.
On January 4, 2021 the Company paid the former members of Infusionz LLC $75,000 as per the acquisition agreement.
On February 1, 2021 the Company issued 182,500 shares of Common Stock in relations to the acquisition of Infusionz LLC. The shares were issued at a $0.85 per common share with adjustments to the final number of shares and value based on the acquisition agreement.
On February 2, 2021 the Company sold the 500,000 shares of Preferred Stock to Allan Marshall, CEO for net proceeds of $50,000.
On February 8, 2021, the Shareholders consented, and the Board of Directors approved the amendment of the Stock Option Plan to increase the maximum number of Shares that may be issued thereunder by 5,000,000 Shares to 10,000,000 Shares.
On February 8, 2021, the Shareholders consented, and the Board of Directors approved the Reverse Stock Split at the rate of 1 share of Common Stock for each 1.8 shares of Common Stock of the Company issued and outstanding (rounded up to the nearest whole number after giving effect to the Reverse Stock Split) on the Record Date of February 5, 2021.
On February 8, 2021, the Board of Directors approved the officers of the Company to file a Registration Statement on Form S-1 (the “Registration Statement”) to be prepared for the purposes of registering (i) up to $20,000,000 of Common Stock at a purchase price of no less than $4.50 per share (post reverse split), including an over-allotment option for the underwriter named therein (the “Underwriter”) to purchase additional shares of Common Stock amounting to 15% of the number of shares of Common Stock offered to the public; and (ii) a warrant to be issued to the Underwriter for the purchase of shares of Common Stock (the “Underwriter Warrant”); and (iii) the shares of Common Stock underlying the Underwriter Warrant (collectively, the “Securities”).
| F-38 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
FINANCIAL STATEMENTS
YEARS ENDED JUNE 30, 2020 AND 2019
| F-39 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Infusionz LLC:
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Infusionz LLC (“the Company”) as of June 30, 2020 and 2019, and the related statements of operations, members’ interest, and cash flows for each of the two years in the period ended June 30, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Infusionz LLC as of June 30, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended June 30, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
We conducted our audits in accordance with auditing standards generally accepted in the United States of America (GAAS). Our responsibilities under those standards are further described in the Auditor's Responsibilities for the Audit of the Financial Statements section of our report. We are required to be independent of Infusionz LLC and to meet our other ethical responsibilities, in accordance with the relevant ethical requirements relating to our audits. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Responsibilities of Management for the Financial Statements
Management is responsible for the preparation and fair presentation of the financial statements in accordance with accounting principles generally accepted in the United States of America, and for the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error. In preparing the financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about Infusionz LLC's ability to continue as a going concern for one year beyond when the financial statements are issued.
Auditor’s Responsibilities for the Audit of the Financial Statements
Our objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor's report that includes our opinion. Reasonable assurance is a high level of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with GAAS will always detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate, they would influence the judgment made by a reasonable user based on the financial statements.
In performing an audit in accordance with GAAS, we:
|
|
· |
Exercise professional judgment and maintain professional skepticism throughout the audit. |
|
|
· |
Identify and assess the risks of material misstatement of the financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. |
|
|
· |
Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of Infusionz LLC's internal control. Accordingly, no such opinion is expressed. |
|
|
· |
Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the financial statements. |
|
|
· |
Conclude whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about Infusionz LLC's ability to continue as a going concern for a reasonable period of time. We are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit, significant audit findings, and certain internal control–related matters that we identified during the audit. |
/s/ B F Borgers CPA PC
We have served as the Company’s auditor since 2019.
Lakewood, Colorado
February 10, 2021
| F-40 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
INFUSIONZ LLC |
|
(In U.S. Dollars, except share data or otherwise stated) |
|
|
|
June 30, |
|
|
June 30, |
| ||
|
|
|
2020 |
|
|
2019 |
| ||
|
ASSETS |
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
| ||
|
Cash |
|
$ | 412,122 |
|
|
$ | 74,473 |
|
|
Accounts receivable, net |
|
|
52,472 |
|
|
|
367,167 |
|
|
Inventory |
|
|
174,845 |
|
|
|
188,345 |
|
|
Prepaid expenses and other current assets |
|
|
8,490 |
|
|
|
- |
|
|
Total current assets |
|
|
647,929 |
|
|
|
629,985 |
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
100,736 |
|
|
|
53,916 |
|
|
Right of use assets |
|
|
29,666 |
|
|
|
- |
|
|
Total assets |
|
$ | 778,331 |
|
|
$ | 683,901 |
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND MEMBERS' EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ | 66,415 |
|
|
$ | 66,400 |
|
|
Accrued liabilities |
|
|
90,876 |
|
|
|
139,569 |
|
|
Deferred revenue |
|
|
112,423 |
|
|
|
44,597 |
|
|
Note payable |
|
|
168,587 |
|
|
|
- |
|
|
Lease obligation |
|
|
29,666 |
|
|
|
- |
|
|
Total current liabilities |
|
|
467,967 |
|
|
|
250,566 |
|
|
|
|
|
|
|
|
|
|
|
|
Note payable |
|
|
212,513 |
|
|
|
- |
|
|
Total liabilities |
|
$ | 680,480 |
|
|
$ | 250,566 |
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies (Note 7) |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Members' equity |
|
|
|
|
|
|
|
|
|
Members' interest |
|
|
28,511 |
|
|
|
28,511 |
|
|
Retained earnings |
|
|
69,340 |
|
|
|
404,824 |
|
|
Total members' equity |
|
|
97,851 |
|
|
|
433,335 |
|
|
Total liabilities and members' equity |
|
$ | 778,331 |
|
|
$ | 683,901 |
|
See accompanying notes to financial statements.
| F-41 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
INFUSIONZ LLC |
|
(In U.S. Dollars, except share data or otherwise stated) |
|
|
|
YEARS ENDED JUNE 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Revenue |
|
|
|
|
|
| ||
|
Product sales |
|
$ | 3,787,495 |
|
|
$ | 3,615,935 |
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
2,837,571 |
|
|
|
2,407,148 |
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
949,924 |
|
|
|
1,208,787 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses |
|
|
1,279,668 |
|
|
|
842,363 |
|
|
|
|
|
1,279,668 |
|
|
|
842,363 |
|
|
|
|
|
|
|
|
|
|
|
|
Other expense (income), net |
|
|
|
|
|
|
|
|
|
Interest expense (income), net |
|
|
5,740 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income |
|
$ | (335,484 | ) |
|
$ | 366,424 |
|
See accompanying notes to financial statements.
| F-42 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
INFUSIONZ LLC |
|
(In U.S. Dollars, except share data or otherwise stated) |
|
|
|
|
|
|
|
|
|
Total |
| |||
|
|
|
Members' |
|
|
Retained |
|
|
Members' |
| |||
|
|
|
Interest |
|
|
Earnings |
|
|
Equity |
| |||
|
2019 |
|
|
|
|
|
|
|
|
| |||
| Balance, June 30, 2019 |
|
|
28,511 |
|
|
$ | 404,824 |
|
|
$ | 433,335 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
- |
|
|
|
(335,484 | ) |
|
|
(335,484 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, June 30, 2020 |
|
$ | 28,511 |
|
|
$ | 69,340 |
|
|
$ | 97,851 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, June 30, 2018 |
|
|
- |
|
|
$ | 38,400 |
|
|
$ | 38,400 |
|
| Member contribution |
|
|
28,511 |
|
|
|
- |
|
|
|
28,511 |
|
| Net income |
|
|
- |
|
|
|
366,424 |
|
|
|
366,424 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, June 30, 2019 |
|
|
28,511 |
|
|
$ | 404,824 |
|
|
$ | 433,335 |
|
See accompanying notes to financial statements.
| F-43 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
INFUSIONZ LLC |
|
(In U.S. Dollars, except share data or otherwise stated) |
|
|
|
YEARS ENDED JUNE 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
| Cash flows from operating activities |
|
|
|
|
|
| ||
|
Net (loss) income |
|
$ | (335,484 | ) |
|
$ | 366,424 |
|
|
Adjustments to reconcile net income to net cash provided by operating activities |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
58,125 |
|
|
|
4,342 |
|
|
Provision for doubtful accounts |
|
|
- |
|
|
|
35,142 |
|
|
Changes in assets and liabilities |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
314,695 |
|
|
|
(359,125 | ) |
|
Inventory |
|
|
13,500 |
|
|
|
(188,275 | ) |
|
Other assets and deposits |
|
|
(61,750 | ) |
|
|
- |
|
|
Accounts payable and accrued liabilities |
|
|
(48,678 | ) |
|
|
171,683 |
|
|
Deferred revenue |
|
|
67,826 |
|
|
|
44,597 |
|
|
Net cash (used) provided by operating activities |
|
|
8,234 |
|
|
|
74,788 |
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Acquisition of property and equipment |
|
|
(51,685 | ) |
|
|
(50,858 | ) |
|
Net cash used in investing activities |
|
|
(51,685 | ) |
|
|
(50,858 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from member contributions |
|
|
- |
|
|
|
21,111 |
|
|
Proceeds from issuance of notes payable |
|
|
381,100 |
|
|
|
- |
|
|
Net cash provided by financing activities |
|
|
381,100 |
|
|
|
21,111 |
|
|
|
|
|
|
|
|
|
|
|
| Net increase in cash |
|
|
337,649 |
|
|
|
45,041 |
|
| Cash, beginning of period |
|
|
74,473 |
|
|
|
29,432 |
|
|
Cash, end of period |
|
$ | 412,122 |
|
|
$ | 74,473 |
|
|
|
|
|
|
|
|
|
|
|
| Supplemental cash flow disclosures |
|
|
|
|
|
|
|
|
| Cash paid for interest |
|
$ | - |
|
|
$ | - |
|
| Cash paid for income taxes |
|
$ | - |
|
|
$ | - |
|
|
|
|
|
|
|
|
|
|
|
| Non-Cash Investing and Financing Activities: |
|
|
|
|
|
|
|
|
| Member contribution of fixed assets |
|
$ | - |
|
|
$ | 7,400 |
|
See accompanying notes to financial statements.
| F-44 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
1. ORGANIZATION AND BUSINESS
Description of the Business
Infusionz LLC (the “Company”) was formed in the state of Colorado in May 2016. The Company develops, manufactures and markets products based on Hemp-based Cannabidiol (“CBD”) including, but not limited to edibles, tinctures, topicals, capsules and pet products. The Company will also manufacture CBD products for other businesses under their brand and specifications.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP").
Use of Estimates
The preparation of the financial statements in conformity with U.S. GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results may differ from these estimates. Significant estimates include the valuation of inventory and the allowance for doubtful accounts.
Concentrations of Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, are accounts receivable and revenue from individual customers in excess of 10%. See Note 8 for significant customer concentration disclosure.
Fair Value of Financial Instruments
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures,” which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value, and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
| F-45 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company has no assets or liabilities valued at fair value on a recurring basis.
Cash and Cash Equivalents
For purposes of the statements of cash flows, the Company considers amounts held by financial institutions and short-term investments with an original maturity of three months or less when purchased to be cash and cash equivalents. As of June 30, 2020 and 2019, the Company had no cash equivalents.
Accounts Receivable
Generally, the Company requires payment prior to shipment. However, in certain circumstances, the Company extends credit terms of 10 to 30 days after shipment to companies located throughout the U.S. Accounts receivable consists of trade accounts arising in the normal course of business. Accounts for which no payments have been received after 30 days from product shipment are considered delinquent and customary collection efforts are initiated. Accounts receivable are carried at original invoice amount less a reserve made for doubtful receivables based on a review of all outstanding amounts on a quarterly basis.
Management has determined the allowance for doubtful accounts by regularly evaluating individual customer receivables and considering a customer’s financial condition and credit history, and current economic conditions. As of each June 30, 2020 and 2019, the Company maintained an allowance for doubtful accounts related to accounts receivable in the amount of $15,000 and $35,142, respectively.
Inventory
Inventory is stated at lower of cost or net realizable value, with cost being determined on a weighted average cost basis. Cost includes costs directly related to manufacturing and distribution of the products. Primary costs include raw materials, packaging, manufacturing overhead, shipping and depreciation of manufacturing equipment and production facilities. Manufacturing overhead includes payroll, employee benefits, utilities, maintenance and property taxes.
The Company performs an assessment of inventory obsolescence to measure inventory at the lower of cost or net realizable value. Factors considered in the determination of obsolescence include slow-moving or non-marketable items.
| F-46 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
Property & Equipment
Property and equipment are stated at cost less accumulated depreciation and impairment, if applicable. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use. Depreciation is provided on a straight-line basis over the assets estimated useful lives, ranging from 2 to 7 years. Tenant improvements are amortized on a straight-line basis over the shorter of the useful life or the remaining life of the related lease. Maintenance or repairs are charged to expense as incurred. Upon sale or disposition, the historically recorded asset cost and accumulated depreciation are removed from the respective accounts and any related gain or loss is recognized.
Impairment of Long-Lived Assets
In accordance with ASC Topic 360, Accounting for the Impairment or Disposal of Long-Lived Assets, the Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of property and equipment is measured by comparing its carrying value to the undiscounted projected future cash flows that the asset(s) are expected to generate. If the carrying amount of an asset is not recoverable, the Company recognizes an impairment loss based on the excess of the carrying amount of the long-lived asset over its respective fair value, which is generally determined as the present value of estimated future cash flows or at the appraised value. The impairment analysis is based on significant assumptions of future results made by management, including revenue and cash flow projections. Circumstances that may lead to impairment of property and equipment include a significant decrease in the market price of a long-lived asset, a significant adverse change in the extent or manner in which a long-lived asset is being used or in its physical condition and a significant adverse change in legal factors or in the business climate that could affect the value of a long-lived asset including an adverse action or assessment by a regulator. As of June 30, 2020 and 2019, the Company determined that long-lived assets were not impaired.
Revenue Recognition
The Company follows the guidance of the Accounting Standards Codification (“ASC”) Topic 606, “Revenue from Contracts with Customers” which is effective as of the annual reporting period beginning after December 15, 2017 using either of two methods: (1) retrospective application of Topic 606 to each prior reporting period presented with the option to elect certain practical expedients as defined within Topic 606 or (2) retrospective application of Topic 606 with the cumulative effect of initially applying Topic 606 recognized at the date of initial application and providing certain additional disclosures as defined per Topic 606. We adopted Topic 606 pursuant to the method (2) and we determined that any cumulative effect for the initial application did not require an adjustment to retained earnings at July 1, 2018.
Most of the Company's revenue contracts represent a single performance obligation related to the fulfillment of customer orders for the purchase of its CBD products. Net sales reflect the transaction prices for these contracts based on the Company's selling list price, which is then reduced by estimated costs for trade promotional programs, consumer incentives, and allowances and discounts used to incentivize sales growth and build brand awareness.
| F-47 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
Revenue is recognized based on the following five step model:
|
- |
Identification of the contract with a customer |
|
- |
Identification of the performance obligations in the contract |
|
- |
Determination of the transaction price |
|
- |
Allocation of the transaction price to the performance obligations in the contract |
|
- |
Recognition of revenue when, or as, the Company satisfies a performance obligation |
The Company recognizes revenue at the point in time that control of the ordered product is transferred to the customer, which is typically upon shipment to the customer or other customer-designated delivery point. Taxes collected from customers that are remitted to governmental agencies are accounted for on a net basis and not included as revenue.
Sales returns from wholesale customers must be completed within 15 days from the date of purchase and are subject to a restocking fee. E-Commerce product returns must be completed within 30 days of the date of purchase. The Company does not accrue for estimated sales returns as historical sales returns have been minimal.
Shipping and handling fees billed to customers are included in revenue. Shipping and handling fees associated with freight are generally included in cost of revenue.
Deferred Revenue
The Company records deposits as deferred revenue when a customer pays in advance of the Company shipping the product. Once the product is shipped, the deposit is recorded as revenue and the related commissions are paid.
Advertising
The Company supports its products with advertising to build brand awareness of the Company’s various products in addition to other marketing programs executed by the Company’s marketing team. The Company believes the continual investment in advertising is critical to the development and sale of its CBD branded products. Advertising costs of $71,640 and $74,633 were expensed as incurred during the years ended June 30, 2020 and 2019, respectively.
Income Taxes
The Company has elected S Corporation status for federal income tax and Colorado corporation business tax purposes. Under these elections, the Company is not a taxpaying entity for federal and state income tax purposes and, accordingly, no provision has been made for such income taxes, except for a minimum state corporate business tax. The stockholders’ allocable share of the Company’s income or loss is reportable on his or her income tax returns.
Recently Issued Accounting Pronouncements
On January 2017, the FASB issued ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, which clarifies the definition of a business to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The standard will be effective for the Company in the first quarter of 2020. The adoption of this standard does not have a material impact on the consolidated financial statements.
| F-48 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
No other recent accounting pronouncements were issued by FASB and the SEC that are believed by management to have a material impact on the Company's present or future financial statements.
3. ACCOUNTS RECEIVABLE, NET
Accounts receivable consist of the following:
|
|
|
June 30, 2020 |
|
|
June 30, 2019 |
| ||
|
Customer receivables |
|
$ | 54,845 |
|
|
$ | 162,216 |
|
|
Merchant receivable from credit card payments from customers |
|
|
12,627 |
|
|
|
240,093 |
|
|
|
|
|
67,472 |
|
|
|
402,309 |
|
|
Less – Allowance for doubtful accounts |
|
|
(15,000 | ) |
|
|
(35,142 | ) |
|
|
|
$ | 52,472 |
|
|
$ | 367,167 |
|
4. INVENTORY
Inventory as of June 30, 2020 and 2019 was comprised of the following:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Raw materials |
|
$ | 162,383 |
|
|
$ | 131,429 |
|
|
Finished goods |
|
|
12,462 |
|
|
|
56,916 |
|
|
|
|
$ | 174,845 |
|
|
$ | 188,345 |
|
5. PROPERTY AND EQUIPMENT, NET
Property and equipment consist of the following:
|
|
|
June 30, 2020 |
|
|
June 30, 2019 |
| ||
|
Furniture and Fixtures |
|
$ | 4,500 |
|
|
$ | 4,500 |
|
|
Computer equipment |
|
|
2,882 |
|
|
|
2,882 |
|
|
Machinery and equipment |
|
|
107,664 |
|
|
|
41,376 |
|
|
Automobiles |
|
|
9,500 |
|
|
|
9,500 |
|
|
|
|
|
124,546 |
|
|
|
58,258 |
|
|
Less - Accumulated depreciation |
|
|
(23,810 | ) |
|
|
(4,342 | ) |
|
|
|
$ | 100,736 |
|
|
$ | 53,916 |
|
Depreciation expense for the years ended June 30, 2020 and 2019 was $20,215 and $4,342, respectively.
| F-49 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
6. ACCRUED LIABILITIES
Accrued expenses as of June 30, 2020 and 2019 were as follows:
|
|
|
June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Accrued payroll and taxes |
|
$ | 82,849 |
|
|
$ | 136,569 |
|
|
Other accrued liabilities |
|
|
8,027 |
|
|
|
3,000 |
|
|
|
|
$ | 90,876 |
|
|
$ | 139,569 |
|
7. NOTES PAYABLE
During the year ended June 30, 2020, the Company entered into a note payable agreement with third party, for total proceeds of $72,000. The principal and interest of the Note is due on demand and is unsecured. The Note has an interest rate of 5%. Subsequent to year end, the note and accrued interest was paid in full.
During the year ended June 30, 2020, the Company entered into a note payable agreement with a related party for total proceeds of $15,000, of which $12,000 remains outstanding at June 30, 2020. The principal and interest of the Note is due on demand and is unsecured. The Note has an interest rate of 3% and was fully paid subsequent to year end.
On May 13, 2020, the Company entered into a Paycheck Protection Program loan for $297,100 in connection with COVID-19. The promissory note has a fixed payment schedule, commencing seven months following the funding of the note and consisting of seventeen monthly payments of principal and interest, with the principal component of each payment based upon the level of amortization of principal over a two year period from the funding date. A final payment for the unpaid principal and accrued interest will be payable no later than May 13, 2022. The note bears interest at a rate of 1.00% per annum and is deferred for the first six months of the loan. Certain portions of the loan may qualify for loan forgiveness based on the terms of the program.
8. OPERATING LEASES
The Company determines if a contract contains a lease at inception. US GAAP requires that the Company’s leases be evaluated and classified as operating or finance leases for financial reporting purposes. The classification evaluation begins at the commencement date and the lease term used in the evaluation includes the non-cancellable period for which the Company has the right to use the underlying asset, together with renewal option periods when the exercise of the renewal option is reasonably certain and failure to exercise such option will result in an economic penalty. The Company’s real estate leases are classified as operating leases and one equipment lease was classified as a financing lease.
Most real estate leases include one or more options to renew, with renewal terms that generally can extend the lease term for an additional two years. The exercise of lease renewal options is at the Company’s discretion. The Company evaluates renewal options at lease inception and on an ongoing basis and includes renewal options that it is reasonably certain to exercise in its expected lease terms when classifying leases and measuring lease liabilities. Lease agreements generally do not require material variable lease payments, residual value guarantees or restrictive covenants.
| F-50 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
The Company’s leases generally do not provide an implicit rate, and therefore the Company uses its incremental borrowing rate as the discount rate when measuring operating lease liabilities. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease within a particular currency environment. The Company used incremental borrowing rates as of July 1, 2019 for operating leases that commenced prior to that date.
During November 2018, the Company entered into a new lease for equipment that commenced on November 1, 2018 and recorded a right of use asset and corresponding lease liability. Lease expense was $6,744 and $1,686 for the years ended June 30, 2020 and 2019, respectively.
The Company’s weighted average remaining lease term and weighted average discount rate for operating leases as of July 1, 2019 are:
|
Weighted average remaining lease term |
|
43 Months |
| |
|
Weighted average incremental borrowing rate |
|
|
5.0 | % |
For the year ended June 30, 2020, the components of lease expense, included in general and administrative expenses and interest expense in the consolidated statements of operations income, are as follows:
|
Operating lease cost: |
|
|
| |
|
Operating lease cost |
|
$ |
26,388 |
|
|
Amortization of ROU assets |
|
$ |
3,965 |
|
|
Total lease cost |
|
$ |
22,423 |
|
The table below reconciles the undiscounted future minimum lease payments (displayed by year and in the aggregate) under noncancelable operating leases with terms of more than one year to the total operating lease liabilities recognized in the consolidated balance sheet as of June 30, 2020:
|
2021 |
|
|
6,744 |
|
|
2022 |
|
|
6,744 |
|
|
2023 |
|
|
6,744 |
|
|
2024 |
|
|
3,934 |
|
|
Total undiscounted future minimum lease payments |
|
|
24,166 |
|
|
Less: Imputed interest |
|
|
(1,741 | ) |
|
Present value of operating lease obligation |
|
|
22,425 |
|
9. RELATED PARTY TRANSACTIONS
During the year ended June 30, 2019, the Company sold approximately $391,732 of CBD products to Green Rush. Green Rush Network LLC is partially owned by the current CEO and owner of Infusionz LLC.
In addition, on January 1, 2019, Green Rush Network LLC contributed certain assets to the Company for approximately 10% of the ownership in Infusionz LLC. The assets were valued at approximately $7,400.
| F-51 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
INFUSIONZ LLC
NOTES TO FINANCIAL STATEMENTS
During the year ended June 30, 2019, the Company contracted with Thing-A-Magig, LLC for consulting services of approximately $20,273. Thing-A-Magig, LLC is partially owned by a member of Infusionz LLC.
During the year ended June 30, 2019, the Company contracted with Green Everett, LLC to provide sales services of approximately $8,393. Green Everett, LLC is partially owned by a member of Infusionz LLC.
During the years ended June 30, 2020 and 2019, the Company contracted with J Charles Management to provide sales services of approximately $5,934 and $25,698, respectively. J Charles Management is partially owned by a member of Infusionz LLC.
During the years ended June 30, 2020 and 2019, the Company paid expenses to NRW Ventures LLC of approximately $12,560 and $75,766, respectively. NRW Ventures LLC is partially owned by the current CEO and owner of Infusionz LLC.
During the years ended June 30, 2020 and 2019, the Company paid expenses to Reido Distributors of approximately $35,694 and $77,962, respectively. In addition, Reido Distributors loaned the Company $15,000, non-interest bearing and due on demand. Reido Distributors is partially owned by a member of Infusionz LLC.
During the year ended June 30, 2019, the Company paid rent to Green Rush Transportation of approximately $7,815. Green Rush Transportation is partially owned by the current CEO and owner of Infusionz LLC.
The above related party transactions are not necessarily indicative of the amounts and terms that would have been incurred had comparable transactions been entered into with independent parties.
10. SIGNIFICANT CUSTOMERS
The Company had significant customers in each of the year presented. A significant customer is defined as one that makes up ten percent or more of total revenues or ten percent of outstanding accounts receivable balance as of the year end.
Net revenues for the year’s ended June 30, 2020 and 2019 include revenues from significant customers as follows:
|
|
|
Years Ended June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Customer A |
|
|
18 | % |
|
|
24 | % |
|
Customer B |
|
|
14 | % |
|
|
12 | % |
Accounts receivable balances as of June 30, 2020 and 2019 from significant customers are as follows:
|
|
|
Years Ended June 30, |
| |||||
|
|
|
2020 |
|
|
2019 |
| ||
|
Customer A |
|
|
38 | % |
|
|
15 | % |
|
Customer C |
|
|
0 | % |
|
|
22 | % |
|
Customer D |
|
|
21 | % |
|
|
10 | % |
|
Customer E |
|
|
0 | % |
|
|
15 | % |
|
Customer F |
|
|
0 | % |
|
|
10 | % |
11. SUBSEQUENT EVENTS
On July 1, 2020, the Company and the shareholder entered into a Stock Purchase Agreement with Grove, Inc. (“Grove”) to sell 100% of the outstanding stock of the Company. Under the purchase method of accounting, the transaction was valued for accounting purposes at an estimated $3,350,000.
| F-52 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Shares
[LOGO]
GROVE, INC.
Common Stock
PROSPECTUS
, 2021
Until , 2021, (the 25th day after the date of this prospectus), all dealers that buy, sell or trade shares of our Common Stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
| 80 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
|
Securities and Exchange Commission Registration Fee |
|
$ |
[____] |
|
FINRA Fees |
|
|
|
|
Nasdaq Listing Fees |
|
$ |
75,000 |
|
Transfer/Edgar Agent Fees |
|
$ |
[____] |
|
Accounting Fees and Expenses |
|
$ |
[____] |
|
Legal Fees and Expenses |
|
$ |
300,000 |
|
Miscellaneous |
|
|
|
|
Total |
|
$ |
[____] |
All amounts are estimates other than the Commission’s registration fee. We are paying all expenses of the offering listed above.
Item 14. Indemnification of Directors and Officers.
Nevada Law
Section 78.7502 of the Nevada Revised Statutes (“NRS”) permits a corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he:
(a) is not liable pursuant to NRS 78.138, or
(b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
In addition, NRS 78.7502 permits a corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he:
(a) is not liable pursuant to NRS 78.138; or
(b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation.
To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to above, or in defense of any claim, issue or matter, the corporation is required to indemnify him against expenses, including attorneys’ fees, actually and reasonably incurred by him in connection with the defense.
| 81 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
NRS 78.752 allows a corporation to purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses.
Other financial arrangements made by the corporation pursuant to NRS 78.752 may include the following:
(a) the creation of a trust fund;
(b) the establishment of a program of self-insurance;
(c) the securing of its obligation of indemnification by granting a security interest or other lien on any assets of the corporation; and
(d) the establishment of a letter of credit, guaranty or surety.
No financial arrangement made pursuant to NRS 78.752 may provide protection for a person adjudged by a court of competent jurisdiction, after exhaustion of all appeals, to be liable for intentional misconduct, fraud or a knowing violation of law, except with respect to the advancement of expenses or indemnification ordered by a court.
Any discretionary indemnification pursuant to NRS 78.7502, unless ordered by a court or advanced pursuant to an undertaking to repay the amount if it is determined by a court that the indemnified party is not entitled to be indemnified by the corporation, may be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made:
(a) by the shareholders;
(b) by the board of directors by majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding;
(c) if a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion, or
(d) if a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion.
Item. 15 Recent Sales of Unregistered Securities.
Common Stock(1)
During the year ended June 30, 2019, the Company issued and sold 5,676,470 shares of common stock for $0.85 per share for approximate cash proceeds of $4,820,000, net of $150,000 offering costs. The proceeds were used for working capital.
On July 19, 2019, the Company sold 23,530 shares of common stock for $20,000. The proceeds were used for working capital.
On July 1, 2020, the Company issued 2,300,000 shares of common stock in a conversion of all the non-Company owned outstanding shares of the Company’s owned subsidiary. The conversion was at $0.85 per share originally paid to the subsidiary in the issuance of the subsidiary’s common stock.
On July 1 and November 1, 2020 and February 1, 2021, the Company issued an aggregate of 915,000 shares of common stock in relation to the acquisition of Infusionz. The shares were issued at a $0.85 per share with adjustments based on the acquisition agreement.
The issuances of the shares of common stock above were exempt from the registration requirements of the Securities Act, pursuant to the exemption for transactions by an issuer not involved in any public offering under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder and corresponding state securities laws.
Preferred Stock(1)
During the year ended June 30, 2019, an investor purchased 500,000 shares of preferred stock for cash proceeds of $50,000. The proceeds were used for working capital.
On February 2, 2021, the Company issued and sold 500,000 shares of preferred stock to Allan Marshall, the Chief Executive Officer of the Company, for the aggregate net proceeds of $50,000. The proceeds were used for working capital.
The issuances of the shares of preferred stock above were exempt from the registration requirements of the Securities Act, pursuant to the exemption for transactions by an issuer not involved in any public offering under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder and corresponding state securities laws.
(1) Pre-reverse stock split figures
| 82 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Item 16. Exhibits
(a) The exhibits listed in the following Exhibit Index are filed as part of this Registration Statement.
|
Exhibit Number |
|
Description of Exhibit |
|
1.1* |
|
Form of Underwriting Agreement |
|
3.1* |
|
Articles of Incorporation of Registrant, as amended |
|
3.2* |
|
Bylaws of Registrant, as amended |
|
4.1* |
|
Convertible Promissory Note, dated October 3, 2019, issued by Registrant in favor of Jeff M. Bishop |
|
4.2* |
|
Convertible Promissory Note, dated October 3, 2019, issued by Registrant in favor of Kyle Dennis |
|
4.3* |
|
Convertible Promissory Note, dated October 17, 2019, issued by Registrant in favor of Jason Bond |
|
4.4* |
|
Promissory Note, Paycheck Protection Program, dated April 28, 2020, issued by Registrant in favor of Bank of the West |
|
4.5* |
|
Loan Authorization and Agreement, dated May 30, 2020, by and between Registrant and the U.S. Small Business Administration |
|
4.6* |
|
Form of Stock Certificate |
|
5.1* |
|
Opinion of Greenberg Traurig, LLP |
|
10.1*† |
|
Grove, Inc. 2019 Incentive Stock Plan (Amended and Restated as of January 31, 2019) |
|
10.2*† |
|
Form of Nonqualified Stock Option Agreement |
|
10.3* |
|
Securities Purchase Agreement, dated as of April 29, 2019, by and between the Registrant and Allan Marshall |
|
21.1* |
|
Subsidiaries of the Registrant |
|
23.1* |
|
Consent of Auditors |
|
23.2* |
|
Consent of Auditors |
|
23.3* |
|
Consent of Greenberg Traurig, LLP (included in Exhibit 5.1) |
|
24.1* |
|
Powers of Attorney (included on Signature Page) |
__________________
*To be filed by amendment
†Indicates management contract or compensatory plan
| 83 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Item 17. Undertakings.
|
|
(a) |
The undersigned registrant (which we refer to as the “Registrant”) hereby undertakes: |
|
|
(1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement: |
|
|
(i) |
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended; |
|
|
|
|
|
|
(ii) |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the U.S. Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
|
|
|
|
|
|
(iii) |
To include any material information with respect to the plan of distribution not previously disclosed in this Registration Statement or any material change to such information in this Registration Statement. |
|
|
(2) |
That, for the purpose of determining any liability under the Securities Act of 1933, as amended, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
|
|
|
|
|
(3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
|
|
|
|
|
(4) |
That, for the purpose of determining liability under the Securities Act of 1933, as amended, to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| 84 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
|
|
(5) |
That, for the purpose of determining liability of the Registrant under the Securities Act of 1933, as amended, to any purchaser in the initial distribution of the securities: The Registrant undertakes that in a primary offering of securities of the Registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
|
|
(i) |
Any preliminary prospectus or prospectus of the Registrant relating to the offering required to be filed pursuant to Rule 424; |
|
|
|
|
|
|
(ii) |
Any free writing prospectus relating to the offering prepared by or on behalf of the Registrant or used or referred to by the Registrant; |
|
|
|
|
|
|
(iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the Registrant or its securities provided by or on behalf of the Registrant; and |
|
|
|
|
|
|
(iv) |
Any other communication that is an offer in the offering made by the Registrant to the purchaser. |
(b) The undersigned registrant hereby further undertakes to provide to the underwriters at the closing date specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(d) The Registrant hereby further undertakes that:
|
|
(1) |
For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this Registration Statement as of the time it was declared effective. |
|
|
|
|
|
|
(2) |
For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 85 |
| Table of Contents |
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of , State of , on , 2021.
|
GROVE, INC. |
| ||
|
|
| ||
|
|
By: |
|
|
|
|
|
Allan Marshall Chief Executive Officer (Principal Executive Officer) |
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Allan Marshall and Andrew J. Norstrud, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him in any and all capacities, to sign (i) any and all amendments (including post-effective amendments) to this Registration Statement and (ii) any registration statement or post-effective amendment thereto to be filed with the U.S. Securities and Exchange Commission pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
|
|
Chief Executive Officer and Chairman of the Board |
|
, 2021 |
|
Allan Marshall |
|
(Principal Executive Officer) |
|
|
|
|
|
|||
|
|
|
|
|
, 2021 |
|
Robert Hackett |
|
President |
|
|
|
|
|
|
|
|
|
|
|
Chief Financial Officer and Director |
|
, 2021 |
|
Andrew J. Norstrud |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|||
|
|
|
Director |
|
, 2021 |
|
Gene Salkind |
|
|
|
|
|
|
|
|||
|
|
|
Director |
|
, 2021 |
|
Thomas C. Williams |
|
|
|
|
|
|
|
|||
|
|
|
Director |
|
, 2021 |
|
Lawrence H. Dugan |
|
|
|
|
| 86 |